Label: COLACE- docusate sodium capsule, liquid filled
- NDC Code(s): 67618-111-28, 67618-111-42, 67618-111-60
- Packager: Atlantis Consumer Healthcare, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- ASK DOCTOR
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE FORMS & STRENGTHS
- HOW SUPPLIED
-
INACTIVE INGREDIENT
Inactive ingredients
gelatin, glycerin, PEG 400, propylene glycol, sorbitolSoft gels are imprinted with edible dye-free ink.
Dist. by: Atlantis Consumer Healthcare Inc.
Bridgewater, NJ 08807
Questions? 1-833-288-2684
©2023 Atlantis Consumer Healthcare Inc.
Colace is a registered trademark of Atlantis Consumer Healthcare Inc. - DOSAGE & ADMINISTRATION
-
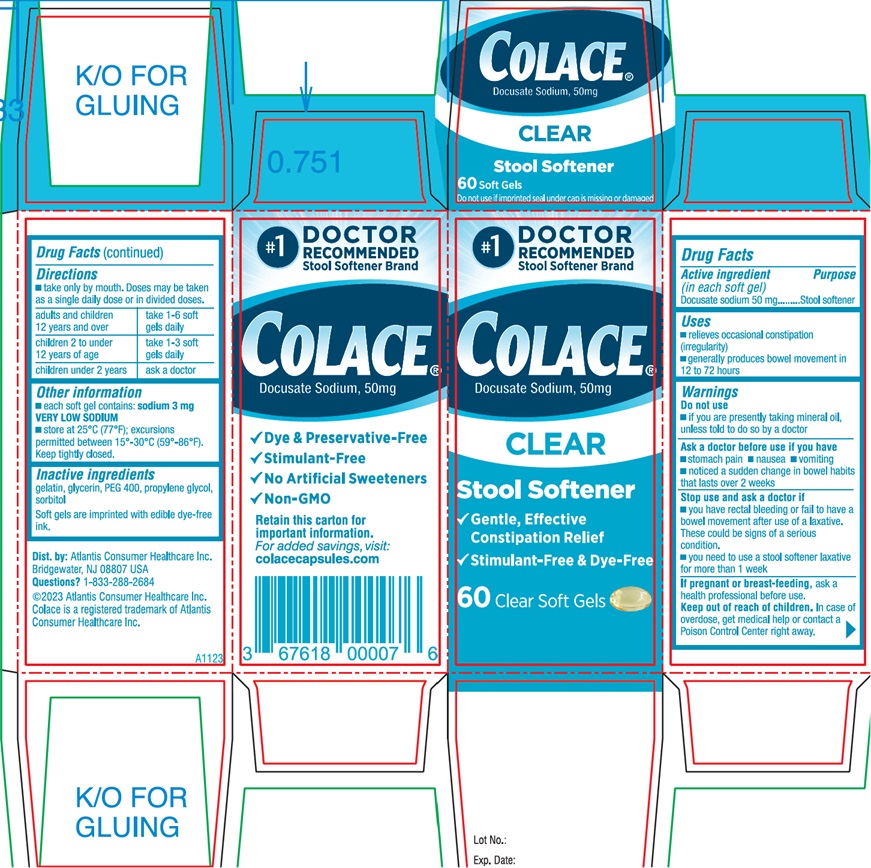
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
#1 DOCTOR
RECOMMENDED
Stool Softener Brand
Colace
Docusate Sodium, 50mg
CLEAR
Stool Softener
√ Gentle, Effective Relief
√ Dye & Preservative-free
√ Cramp & Stimulant-free
28 Clear Soft Gels
Front Panel
#1 DOCTOR
RECOMMENDED
Stool Softener Brand
Colace®
Docusate Sodium, 50mg
CLEAR
Stool Softener
√ Gentle, Effective
Constipation Relief
√ Stimulant-Free & Dye-Free
42 Clear Soft Gels
Side Panel
Colace®
Docusate Sodium, 50mg
√ Dye & Preservative-Free
√ Stimulant-Free
√ No Artificial Sweeteners
√ Non-GMO
Retain this carton for
Important information.
For added savings, visit:
colacecapsules.com
Dist. by: Atlantis Consumer Healthcare Inc.
Bridgewater, NJ 08807 USA
Questions? 1-833-288-2684
©2023 Atlantis Consumer Healthcare Inc.
Colace is a registered trademark of Atlantis
Consumer Healthcare Inc.
Lot No.:
Exp. Date:
A0923
Front Panel
#1 DOCTOR
RECOMMENDED
Stool Softener Brand
Colace®
Docusate Sodium, 50mg
CLEAR
Stool Softener
√ Gentle, Effective
Constipation Relief
√ Stimulant-Free & Dye-Free
60 Clear Soft Gels
Side Panel
Colace®
Docusate Sodium, 50mg
√ Dye & Preservative-Free
√ Stimulant-Free
√ No Artificial Sweeteners
√ Non-GMO
Retain this carton for
Important information.
For added savings, visit:
colacecapsules.com
Dist. by: Atlantis Consumer Healthcare Inc.
Bridgewater, NJ 08807 USA
Questions? 1-833-288-2684
©2023 Atlantis Consumer Healthcare Inc.
Colace is a registered trademark of Atlantis
Consumer Healthcare Inc.
Lot No.:
Exp. Date:
A1123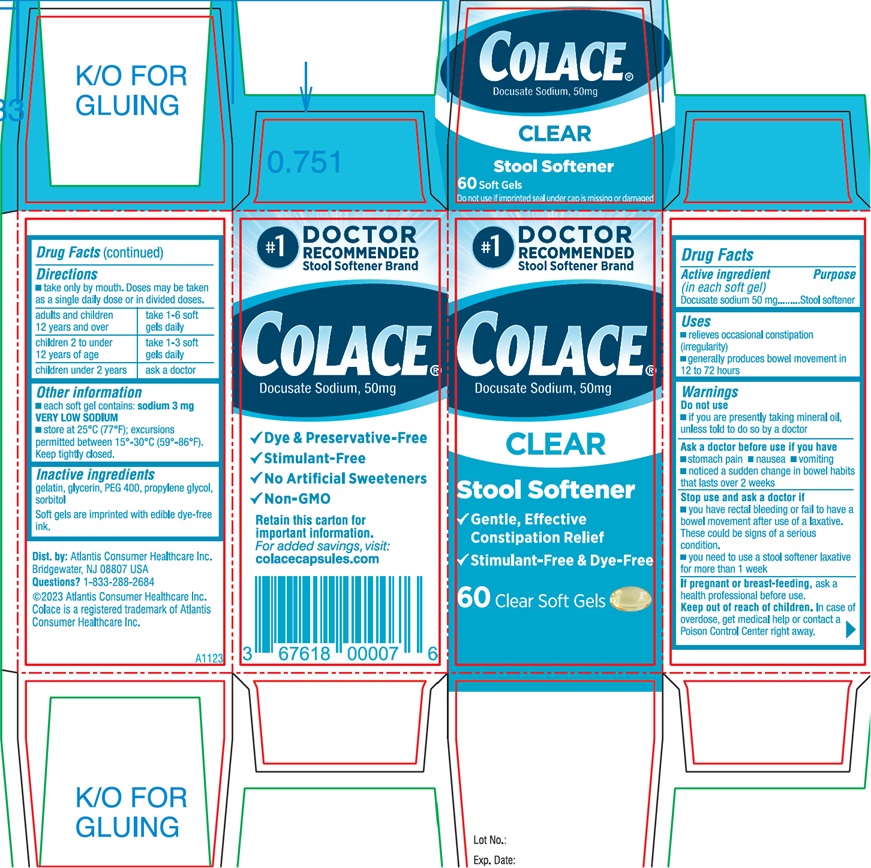
-
INGREDIENTS AND APPEARANCE
COLACE
docusate sodium capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67618-111 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 50 mg Inactive Ingredients Ingredient Name Strength GELATIN, UNSPECIFIED (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SORBITOL (UNII: 506T60A25R) Product Characteristics Color BROWN (clear to light brown) Score no score Shape OVAL Size 11mm Flavor Imprint Code CLR;50 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67618-111-28 1 in 1 CARTON 01/07/1900 1 28 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:67618-111-42 1 in 1 CARTON 02/01/2024 2 42 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:67618-111-60 1 in 1 CARTON 04/01/2024 3 60 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 01/07/1900 Labeler - Atlantis Consumer Healthcare, Inc. (118983925) Registrant - Atlantis Consumer Healthcare, Inc. (118983925)