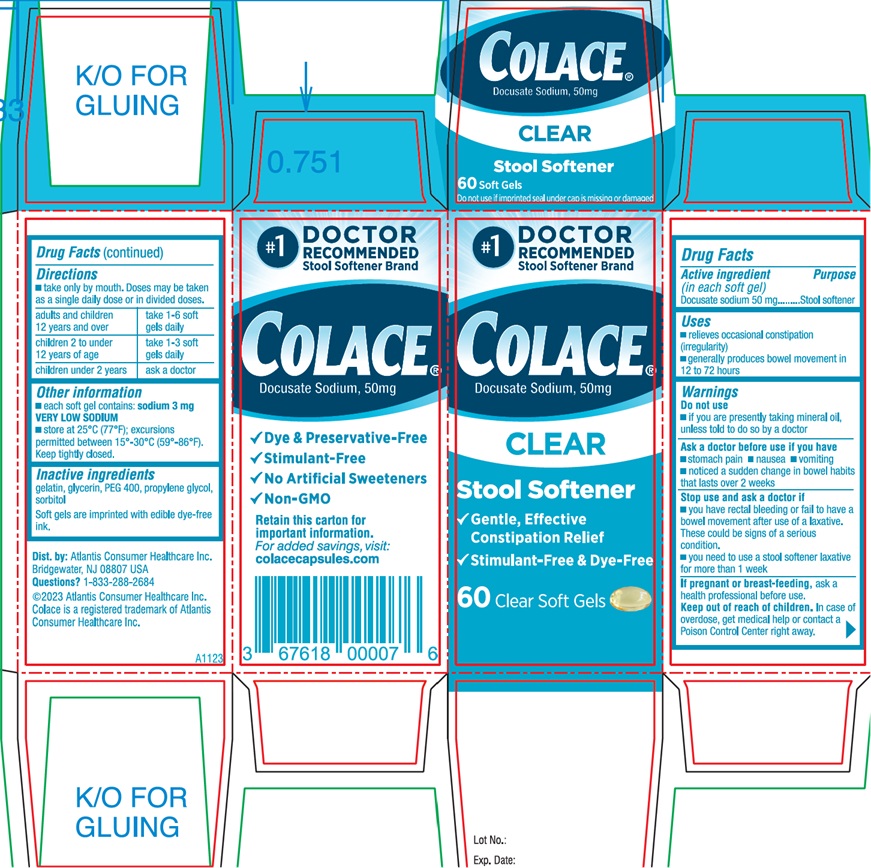
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
- you have rectal bleeding or fail to have a bowel movementafter use of a laxative. These could be signs of a serious condition.
- you need to use a stool softener laxative for more than1 week
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
- Take only by mouth. Doses may be taken as a single daily dose or in divided doses.
| adults and children 12 years and over | take 1-6 soft gels daily |
| children 2 to under 12 years of age | take 1-3 soft gels daily |
| children under 2 years | ask a doctor |
- each soft gel contains: sodium 3 mg VERY LOW SODIUM
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F).
Inactive ingredients
gelatin, glycerin, PEG 400, propylene glycol, sorbitol
Soft gels are imprinted with edible dye-free ink.
Dist. by: Atlantis Consumer Healthcare Inc.
Bridgewater, NJ 08807
Questions? 1-833-288-2684
©2023 Atlantis Consumer Healthcare Inc.
Colace is a registered trademark of Atlantis Consumer Healthcare Inc.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
#1 DOCTOR
RECOMMENDED
Stool Softener Brand
Colace
Docusate Sodium, 50mg
CLEAR
Stool Softener
√ Gentle, Effective Relief
√ Dye & Preservative-free
√ Cramp & Stimulant-free
28 Clear Soft Gels

Front Panel
#1 DOCTOR
RECOMMENDED
Stool Softener Brand
Colace®
Docusate Sodium, 50mg
CLEAR
Stool Softener
√ Gentle, Effective
Constipation Relief
√ Stimulant-Free & Dye-Free
42 Clear Soft Gels
Side Panel
Colace®
Docusate Sodium, 50mg
√ Dye & Preservative-Free
√ Stimulant-Free
√ No Artificial Sweeteners
√ Non-GMO
Retain this carton for
Important information.
For added savings, visit:
colacecapsules.com
Dist. by: Atlantis Consumer Healthcare Inc.
Bridgewater, NJ 08807 USA
Questions? 1-833-288-2684
©2023 Atlantis Consumer Healthcare Inc.
Colace is a registered trademark of Atlantis
Consumer Healthcare Inc.
Lot No.:
Exp. Date:
A0923

Front Panel
#1 DOCTOR
RECOMMENDED
Stool Softener Brand
Colace®
Docusate Sodium, 50mg
CLEAR
Stool Softener
√ Gentle, Effective
Constipation Relief
√ Stimulant-Free & Dye-Free
60 Clear Soft Gels
Side Panel
Colace®
Docusate Sodium, 50mg
√ Dye & Preservative-Free
√ Stimulant-Free
√ No Artificial Sweeteners
√ Non-GMO
Retain this carton for
Important information.
For added savings, visit:
colacecapsules.com
Dist. by: Atlantis Consumer Healthcare Inc.
Bridgewater, NJ 08807 USA
Questions? 1-833-288-2684
©2023 Atlantis Consumer Healthcare Inc.
Colace is a registered trademark of Atlantis
Consumer Healthcare Inc.
Lot No.:
Exp. Date:
A1123