Label: ECOCARE 360- benzalkonium solution
-
NDC Code(s):
47593-275-11,
47593-275-21,
47593-275-30,
47593-275-41, view more47593-275-56, 47593-275-59, 47593-275-83, 47593-275-84
- Packager: Ecolab Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other Information
- INACTIVE INGREDIENT
- Questions?
-
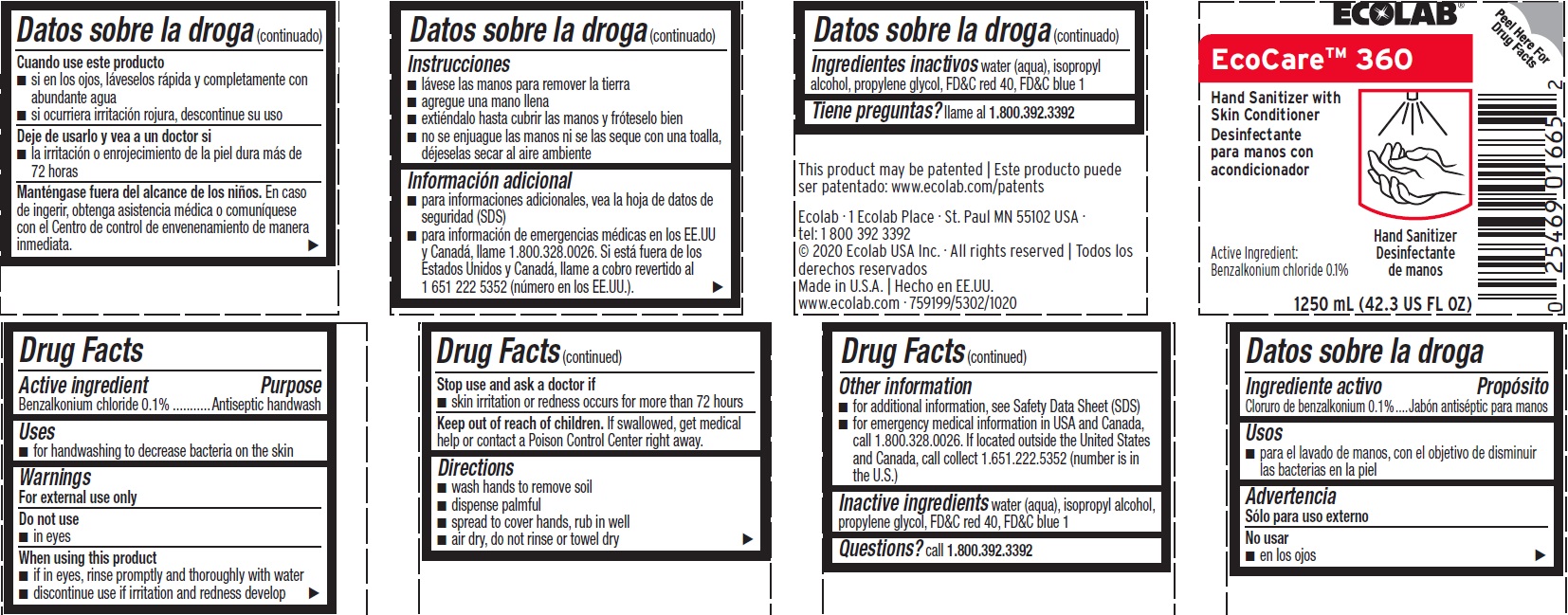
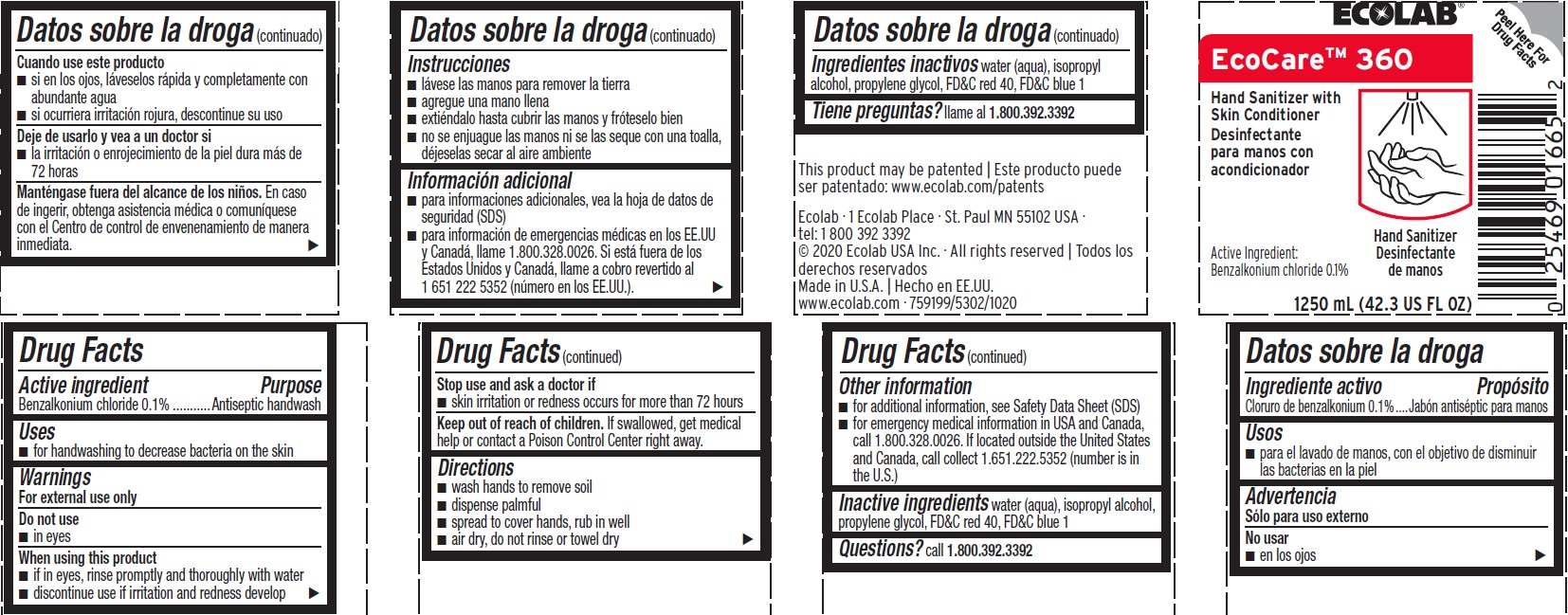
Principal Display Panel - Representative Label
ECOLAB
EcoCare 360
Hand Sanitizer with Skin Conditioner
Active Ingredient: Benzalkonium Chloride 0.1%
1250 mL (42.3 US Fl Oz
Ecolab · 1 Ecolab Place · St. Paul MN 55102 USA
tel: 1 800 392 3392
© 2020 Ecolab USA Inc. · All rights reserved | Todos los derechos reservados
Made in U.S.A. | Hecho en EE.UU.
www.ecolab.com · 759199/5302/1020
-
INGREDIENTS AND APPEARANCE
ECOCARE 360
benzalkonium solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47593-275 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.1 mg in 100 mL Inactive Ingredients Ingredient Name Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47593-275-83 2000 mL in 1 BAG; Type 0: Not a Combination Product 02/23/1999 2 NDC:47593-275-84 8000 mL in 1 BAG; Type 0: Not a Combination Product 02/23/1999 3 NDC:47593-275-11 3780 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/23/1999 4 NDC:47593-275-21 208000 mL in 1 DRUM; Type 0: Not a Combination Product 02/23/1999 5 NDC:47593-275-30 207 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/23/1999 6 NDC:47593-275-41 750 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/23/1999 7 NDC:47593-275-56 1200 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/23/1999 8 NDC:47593-275-59 1250 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/16/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 02/23/1999 Labeler - Ecolab Inc. (006154611)