Warnings
For external use onlyDirections
- Wash hands to remove soil
- Dispense palmful
- Spread to cover hands, rub in well
- Air dry, do not rinse or towel dry
Other Information
- for additional information, see Safety Data Sheet (SDS)
- for emergency medical information in USA and Canada, call 1.800.328.0026. If located outside the United States and Canada, call collect 1.651.222.5352 (number is in the U.S.)
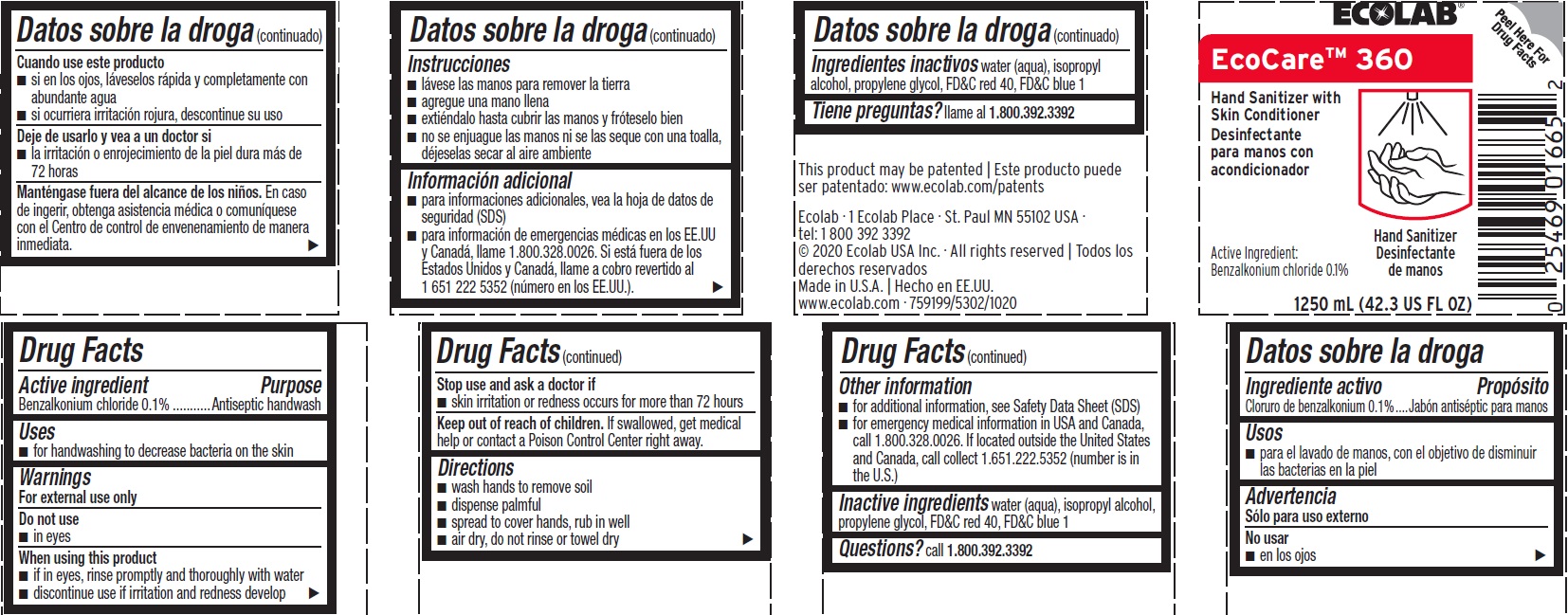
Principal Display Panel - Representative Label
ECOLAB
EcoCare 360
Hand Sanitizer with Skin Conditioner
Active Ingredient: Benzalkonium Chloride 0.1%
1250 mL (42.3 US Fl Oz
Ecolab · 1 Ecolab Place · St. Paul MN 55102 USA
tel: 1 800 392 3392
© 2020 Ecolab USA Inc. · All rights reserved | Todos los derechos reservados
Made in U.S.A. | Hecho en EE.UU.
www.ecolab.com · 759199/5302/1020