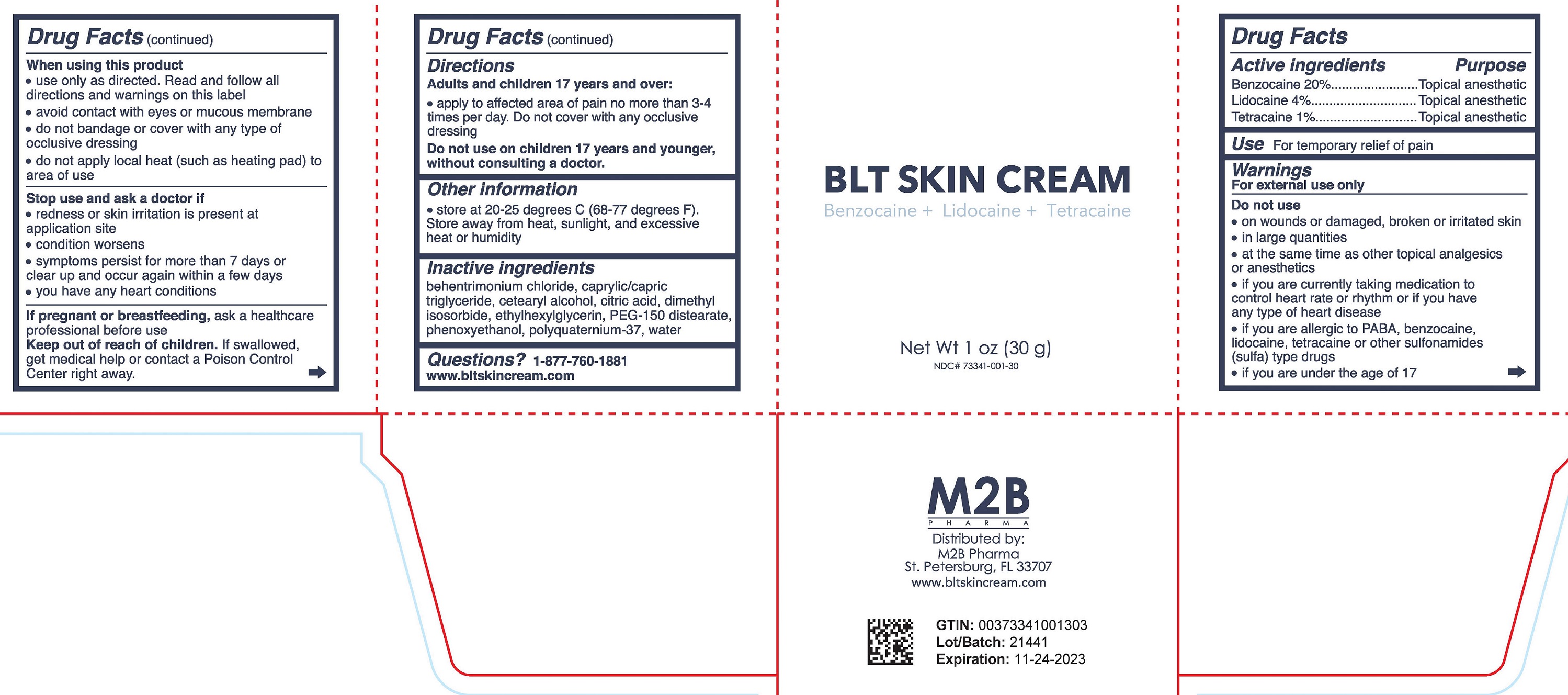
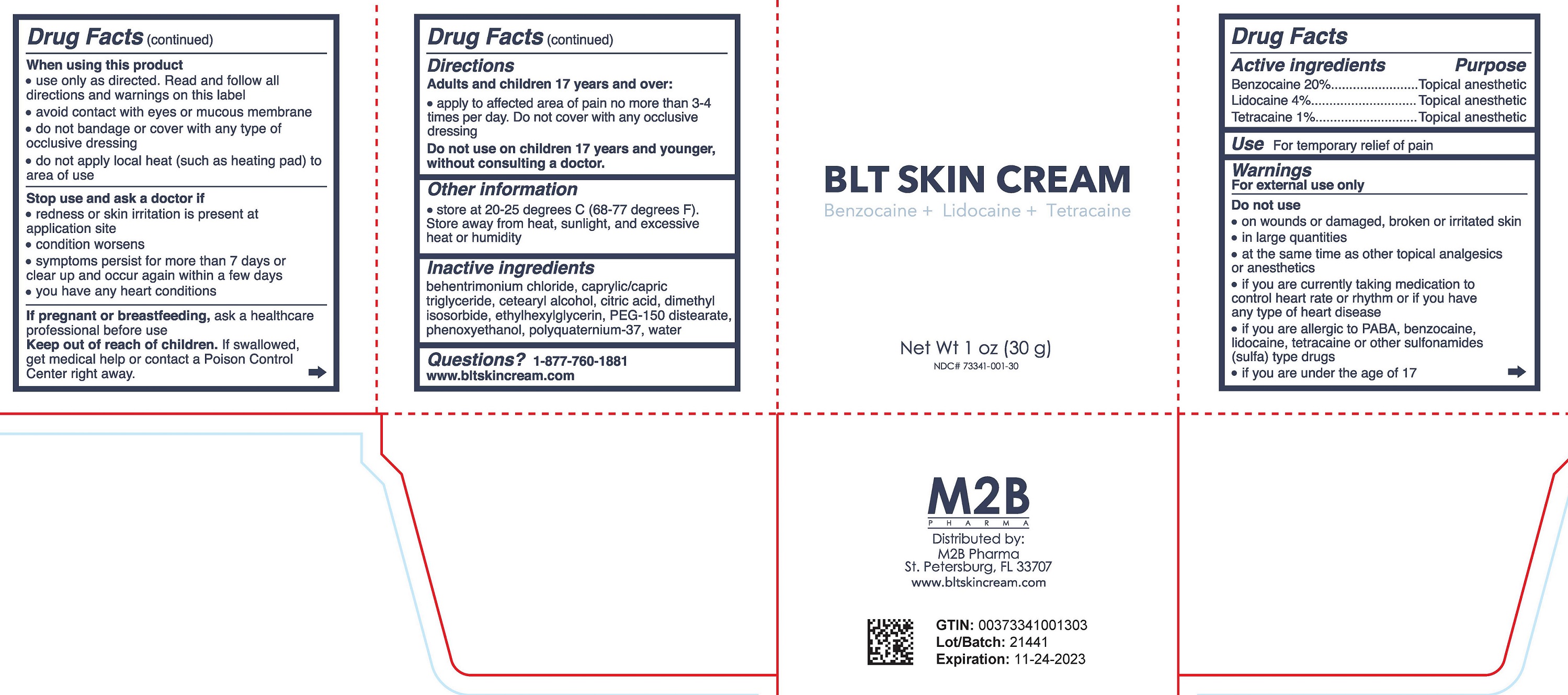
Label: BLT SKIN CREAM- benzocaine, lidocaine, tetracaine cream
- NDC Code(s): 73341-001-01, 73341-001-30
- Packager: M2B Pharma LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTS
- Active ingredient
- Purpose
- Active ingredient
- Purpose
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- On wounds or damaged, broken or irritated skin
- In large quantities
- At the same time as other topical analgesics or anesthetics
- If you are currently taking medication to control heart rate or rhythm or if you have any type of heart disease
- If you are allergic to PABA, benzocaine, lidocaine, tetracaine or other sulfonamides (sulfa) type drugs
- If you are under the age of 17
When using this product
- Use only as directed. Read and follow all directions and warnings on this label.
- Avoid contact with eyes or mucous membrane
- Do not bandage or cover with any type of clear occlusive dressing
- Do not apply local heat (such as heating pad) to area of use
- Directions
- Other Information
- Inactive Ingredients
- Questions?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
BLT SKIN CREAM
benzocaine, lidocaine, tetracaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73341-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 0.2 g in 1 g TETRACAINE (UNII: 0619F35CGV) (TETRACAINE - UNII:0619F35CGV) TETRACAINE 0.01 g in 1 g LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 0.04 g in 1 g Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) POLYQUATERNIUM 37 (200 MPA.S) (UNII: 67C1D6YV24) BEHENTRIMONIUM CHLORIDE (UNII: X7GNG3S47T) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) WATER (UNII: 059QF0KO0R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PEG-150 DISTEARATE (UNII: 6F36Q0I0AC) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73341-001-30 30 g in 1 JAR; Type 0: Not a Combination Product 02/04/2020 2 NDC:73341-001-01 454 g in 1 JAR; Type 0: Not a Combination Product 12/23/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 02/04/2020 Labeler - M2B Pharma LLC (117182771)