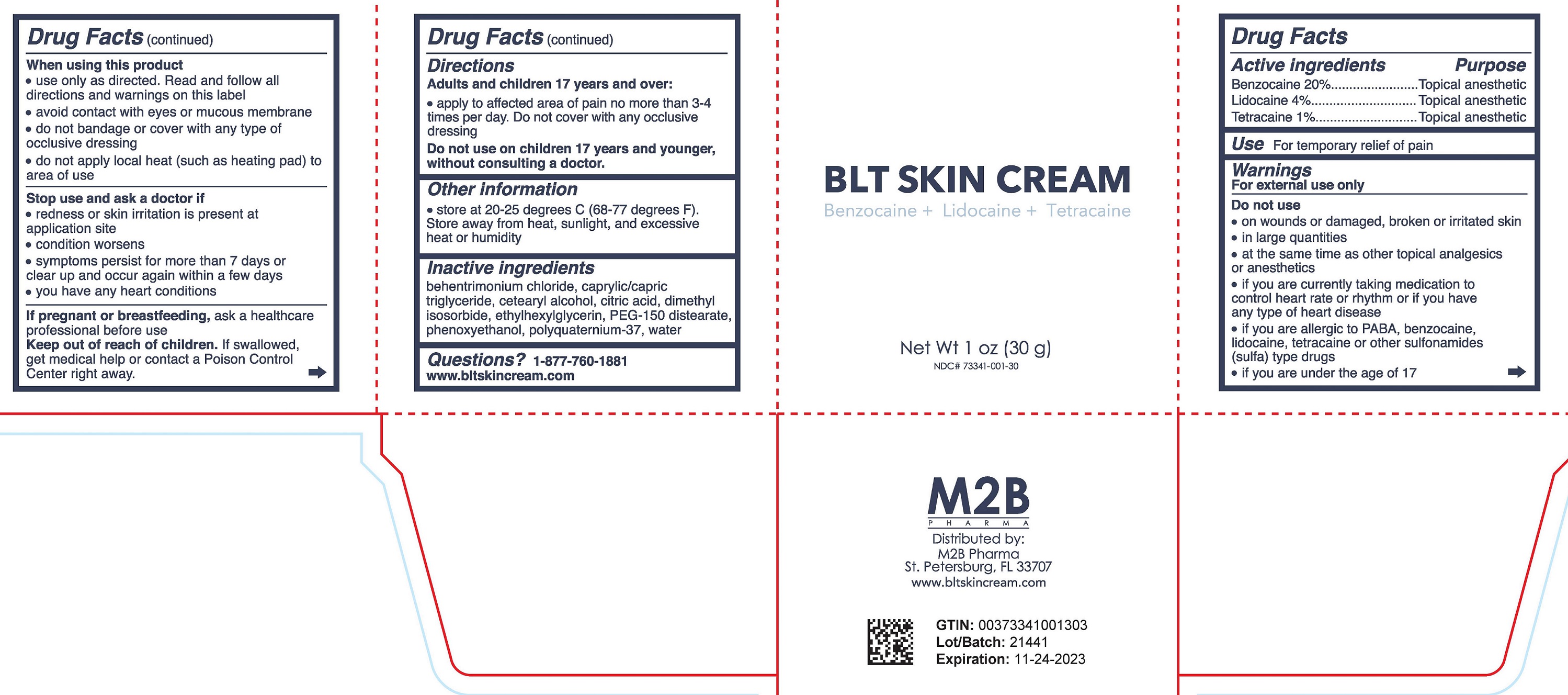
Warnings
For external use only
Do not use
- On wounds or damaged, broken or irritated skin
- In large quantities
- At the same time as other topical analgesics or anesthetics
- If you are currently taking medication to control heart rate or rhythm or if you have any type of heart disease
- If you are allergic to PABA, benzocaine, lidocaine, tetracaine or other sulfonamides (sulfa) type drugs
- If you are under the age of 17
When using this product
- Use only as directed. Read and follow all directions and warnings on this label.
- Avoid contact with eyes or mucous membrane
- Do not bandage or cover with any type of clear occlusive dressing
- Do not apply local heat (such as heating pad) to area of use
Directions
Adults and children over 17 years of age:
- Apply to affected area of pain no more than 3-4 times per day. Do not cover with any occlusive dressing
Do not use on children 17 years and younger without consulting a doctor.
Other Information
- Store at 20-25 degrees C (68-77 degrees F).
- Store away from heat, sunlight, and excessive heat or humidity