Label: ASEPXIA SOFTENING ACNE BAR- salicylic acid soap
- NDC Code(s): 50066-805-01
- Packager: Genomma Lab USA
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Uses
-
Warnings
For external use only
, skin irritation and dryness may occur if you use other topical acne medications at the same time or immediately following use of this product. If this occurs, use only one medication at a time unless directed by a doctor. Avoid contact with eyes. If product gets in eyes, rinse with water. When using this product
- Directions
- Other information
-
Inactive ingredients
Sodium palmate, Sodium cocoate and/or Sodium palm kernelate, Water, Glycerin, Fragrance, Sodium laureth sulfate, Sodium hydroxide, Sodium chloride, Glycereth-26, Tetrasodium etidronate, Glycolic acid, Mineral oil, Titanium dioxide, Cucumis sativus (Cucumber) fruit extract, Stearic Acid, Cetyl alcohol, Glyceryl stearate, Salix alba (White Willow) bark extract, FD&C Blue 1, Citric acid, Disodium EDTA, Triethanolamine, Opuntia Ficus-Indica (Cactus) stem extract, Carbomer, D&C Red 33, Iron Oxide, Pentasodium pentetate
- QUESTIONS
- SPL UNCLASSIFIED SECTION
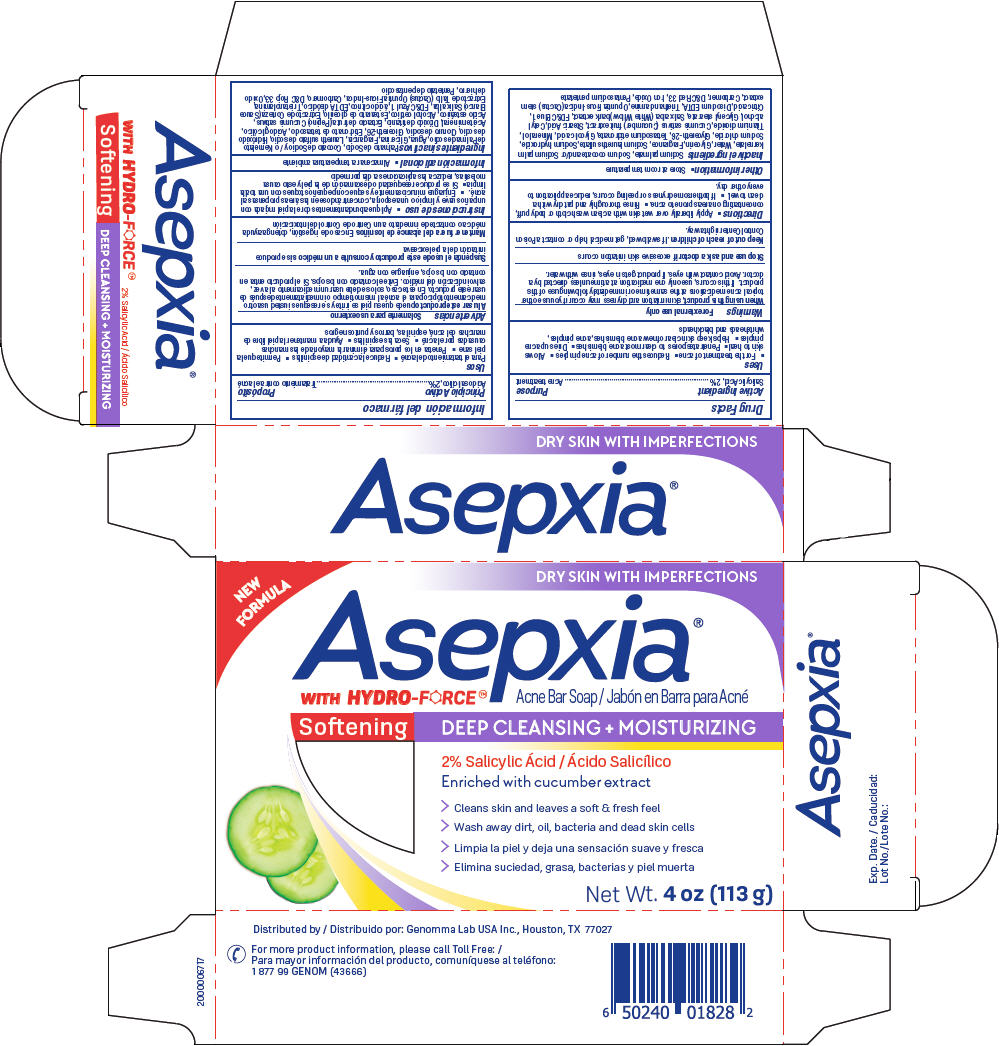
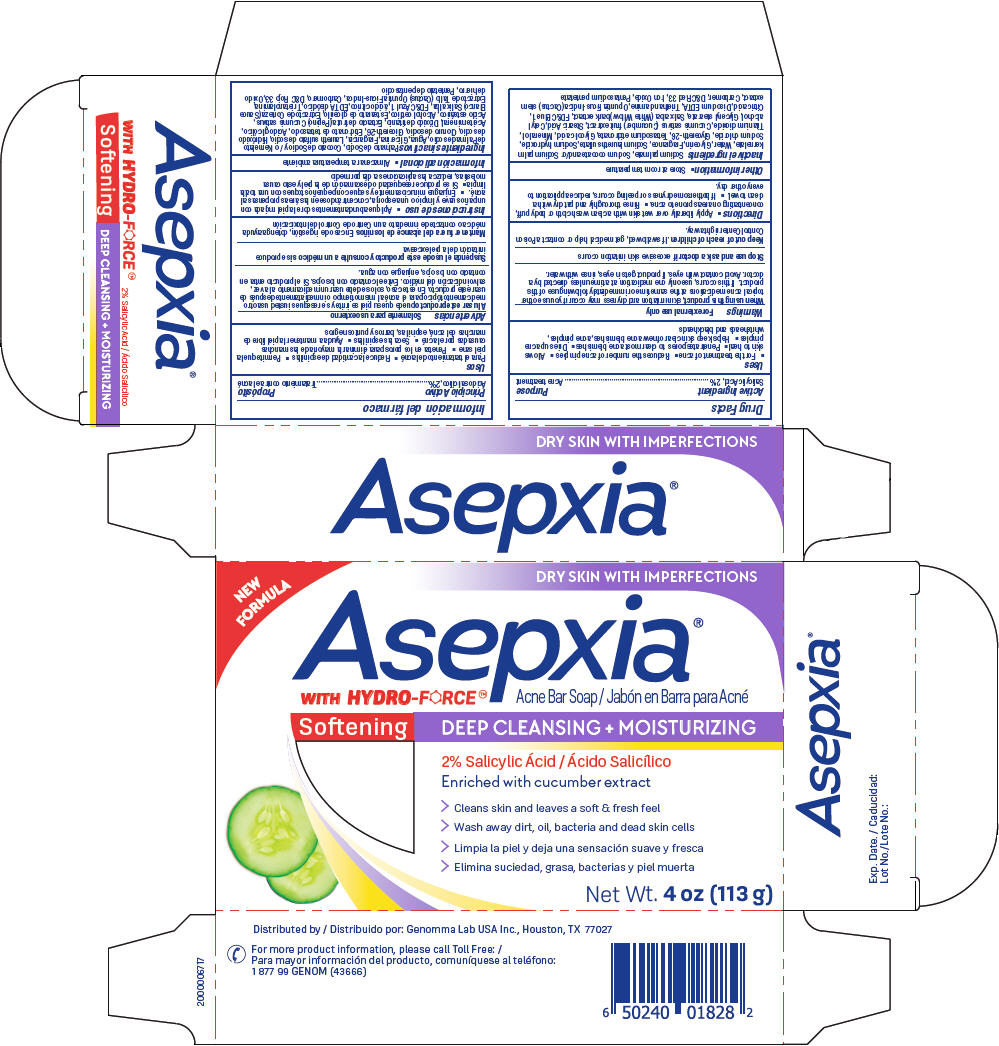
- PRINCIPAL DISPLAY PANEL - 113 g Bar Carton
-
INGREDIENTS AND APPEARANCE
ASEPXIA SOFTENING ACNE BAR
salicylic acid soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50066-805 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 g in 100 g Inactive Ingredients Ingredient Name Strength GLYCOLIC ACID (UNII: 0WT12SX38S) MINERAL OIL (UNII: T5L8T28FGP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) CUCUMBER (UNII: YY7C30VXJT) STEARIC ACID (UNII: 4ELV7Z65AP) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) TROLAMINE (UNII: 9O3K93S3TK) OPUNTIA FICUS-INDICA STEM (UNII: MUD8892KHL) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FERRIC OXIDE RED (UNII: 1K09F3G675) PENTASODIUM PENTETATE (UNII: 961TOZ5L7T) SODIUM PALMATE (UNII: S0A6004K3Z) SODIUM COCOATE (UNII: R1TQH25F4I) SODIUM PALM KERNELATE (UNII: 6H91L1NXTW) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) SODIUM LAURETH-3 SULFATE (UNII: BPV390UAP0) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM CHLORIDE (UNII: 451W47IQ8X) GLYCERETH-26 (UNII: NNE56F2N14) ETIDRONATE TETRASODIUM (UNII: CZZ9T1T1X4) Product Characteristics Color purple Score Shape Size Flavor Imprint Code Asepxia Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50066-805-01 1 in 1 CARTON 03/19/2020 1 113 g in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 03/19/2020 Labeler - Genomma Lab USA (832323534)