Uses
- For the treatment of acne
- Reduces the number of acne pimples
- Allows skin to heal
- Penetrates pores to clear most acne blemishes
- Dries up acne pimples
- Helps keep skin clear of new acne blemishes, acne pimples, whiteheads and blackheads
Warnings
For external use only
, skin irritation and dryness may occur if you use other topical acne medications at the same time or immediately following use of this product. If this occurs, use only one medication at a time unless directed by a doctor. Avoid contact with eyes. If product gets in eyes, rinse with water. When using this product
Directions
- Apply liberally over wet skin with a clean wash cloth or body puff, concentrating on areas prone to acne.
- Rinse thoroughly and pat dry with a clean towel.
- If bothersome dryness or peeling occurs, reduce application to every other day.
Inactive ingredients
Sodium palmate, Sodium cocoate and/or Sodium palm kernelate, Water, Glycerin, Fragrance, Sodium laureth sulfate, Sodium hydroxide, Sodium chloride, Glycereth-26, Tetrasodium etidronate, Glycolic acid, Mineral oil, Titanium dioxide, Cucumis sativus (Cucumber) fruit extract, Stearic Acid, Cetyl alcohol, Glyceryl stearate, Salix alba (White Willow) bark extract, FD&C Blue 1, Citric acid, Disodium EDTA, Triethanolamine, Opuntia Ficus-Indica (Cactus) stem extract, Carbomer, D&C Red 33, Iron Oxide, Pentasodium pentetate
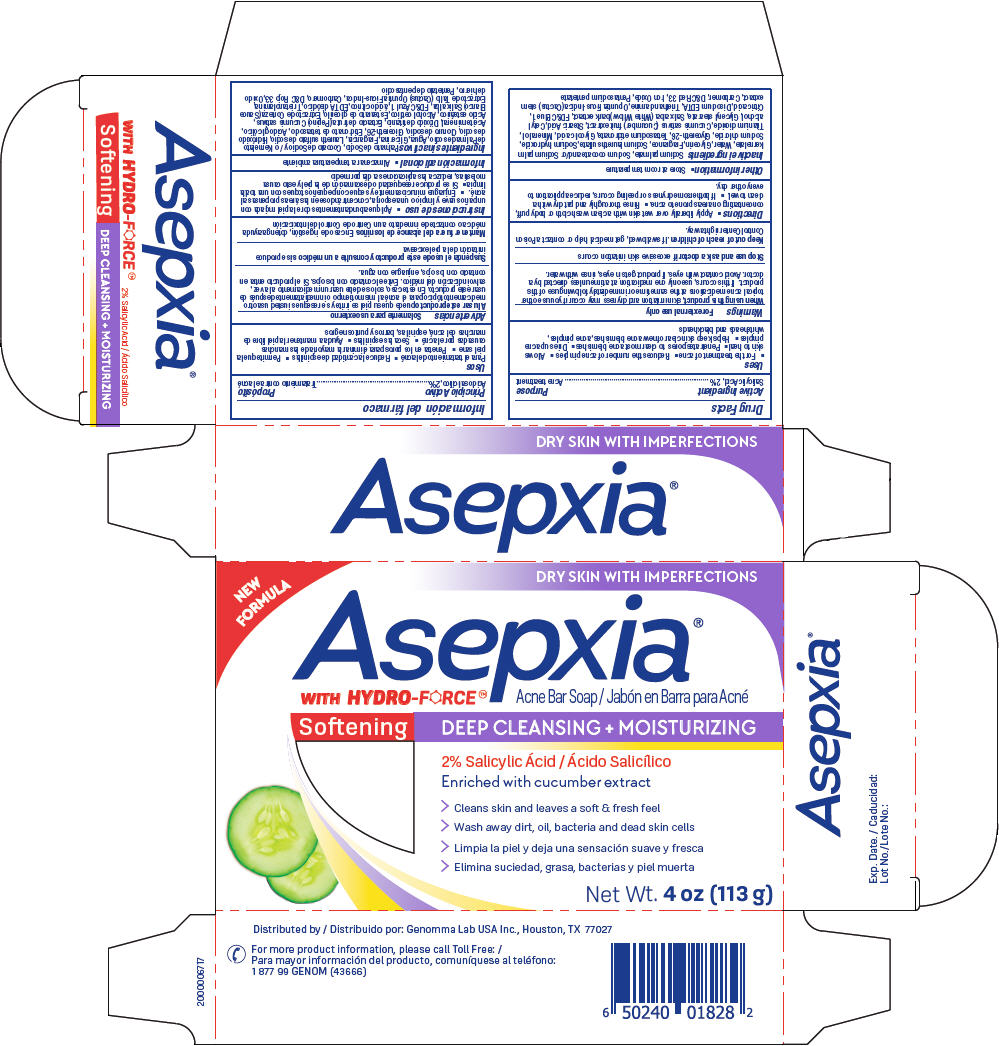
PRINCIPAL DISPLAY PANEL - 113 g Bar Carton
NEW FORMULA
DRY SKIN WITH IMPERFECTIONS
Acne Bar Soap
AsepxiaWITH HYDRO-FORCE®®
Softening DEEP CLEANSING + MOISTURIZING
2% Salicylic Ácid
Enriched with cucumber extract
> Cleans skin and leaves a soft & fresh feel
> Wash away dirt, oil, bacteria and dead skin cells
Net Wt. 4 oz (113 g)