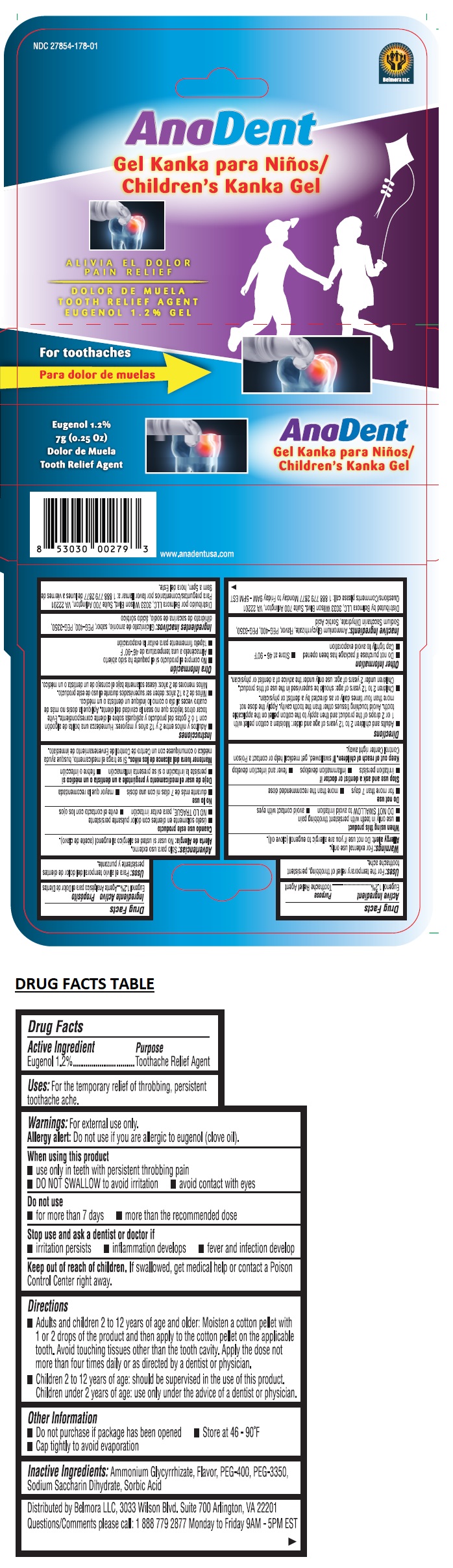
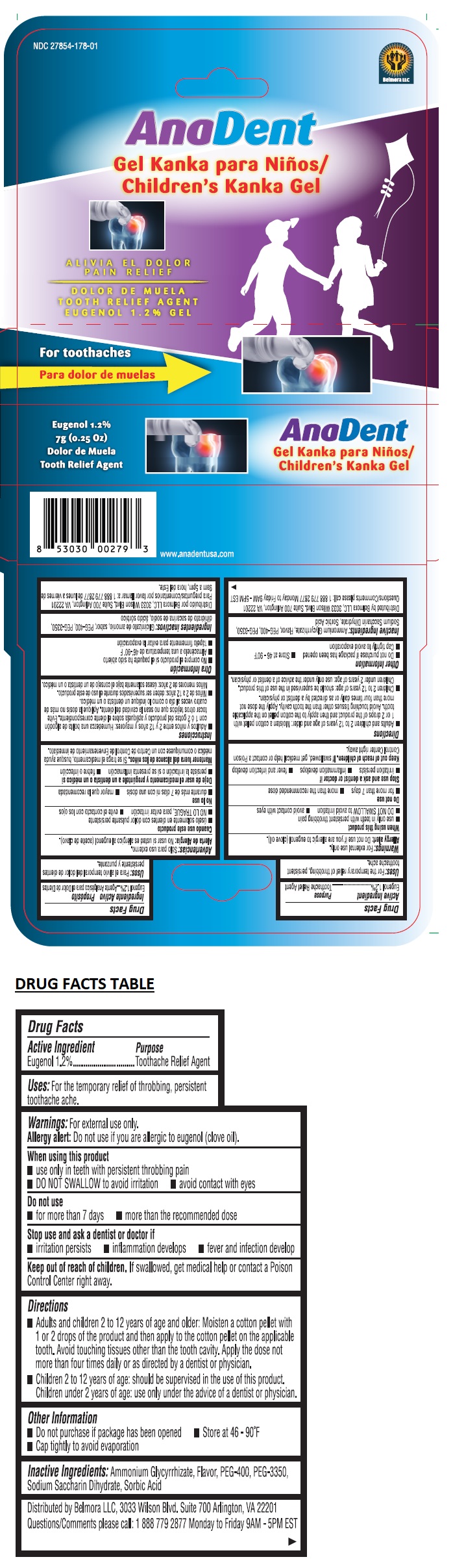
Label: ANADENT CHILDRENS KANKA GEL- eugenol gel
- NDC Code(s): 27854-178-01
- Packager: Belmora LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 8, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Purpose
- Uses:
-
Warnings:
For external use only.
Allergy alert: Do not use if you are allergic to eugenol (clove oil).
When using this product
• use only in teeth with persistent throbbing pain
• DO NOT SWALLOW to avoid irritation • avoid contact with eyesDo not use
• for more than 7 days • more than the recommended doseStop use and ask a dentist or doctor if
• irritation persists • inflammation develops • fever and infection develop -
Directions
• Adults and children 2 to 12 years of age and older: Moisten a cotton pellet with 1 or 2 drops of the product and then apply to the cotton pellet on the applicable tooth. Avoid touching tissues other than the tooth cavity. Apply the dose not more than four times daily or as directed by a dentist or physician.
• Children 2 to 12 years of age: should be supervised in the use of this product. Children under 2 years of age: use only under the advice of a dentist or physician. - Other Information
- Inactive Ingredients:
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- Packaging
-
INGREDIENTS AND APPEARANCE
ANADENT CHILDRENS KANKA GEL
eugenol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:27854-178 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EUGENOL (UNII: 3T8H1794QW) (EUGENOL - UNII:3T8H1794QW) EUGENOL 12 mg in 1 g Inactive Ingredients Ingredient Name Strength AMMONIUM GLYCYRRHIZATE (UNII: 3VRD35U26C) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SORBIC ACID (UNII: X045WJ989B) Product Characteristics Color Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:27854-178-01 1 in 1 CARTON 11/08/2022 1 7 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 11/08/2022 Labeler - Belmora LLC (112753244)