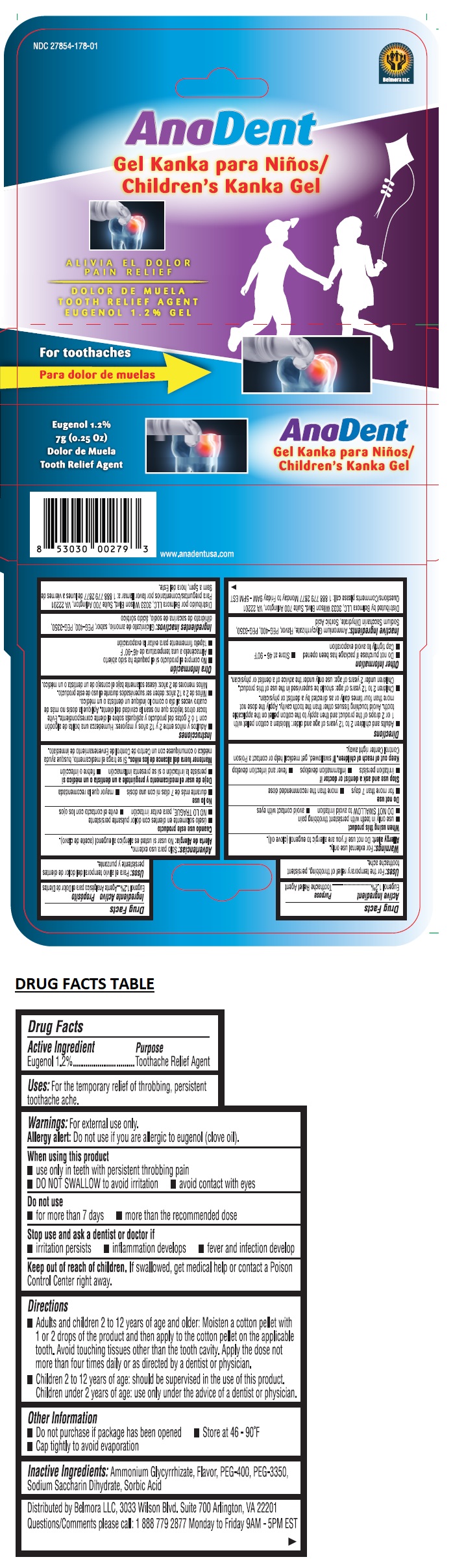
Warnings:
For external use only.
Allergy alert: Do not use if you are allergic to eugenol (clove oil).
When using this product
• use only in teeth with persistent throbbing pain
• DO NOT SWALLOW to avoid irritation • avoid contact with eyes
Do not use
• for more than 7 days • more than the recommended dose
Stop use and ask a dentist or doctor if
• irritation persists • inflammation develops • fever and infection develop
Directions
• Adults and children 2 to 12 years of age and older: Moisten a cotton pellet with 1 or 2 drops of the product and then apply to the cotton pellet on the applicable tooth. Avoid touching tissues other than the tooth cavity. Apply the dose not more than four times daily or as directed by a dentist or physician.
• Children 2 to 12 years of age: should be supervised in the use of this product. Children under 2 years of age: use only under the advice of a dentist or physician.
Other Information
• Do not purchase if package has been opened • Store at 46 - 90°F
• Cap tightly to avoid evaporation
Inactive Ingredients:
Ammonium Glycyrrhizate, Flavor, PEG-400, PEG-3350, Sodium Saccharin Dihydrate, Sorbic Acid