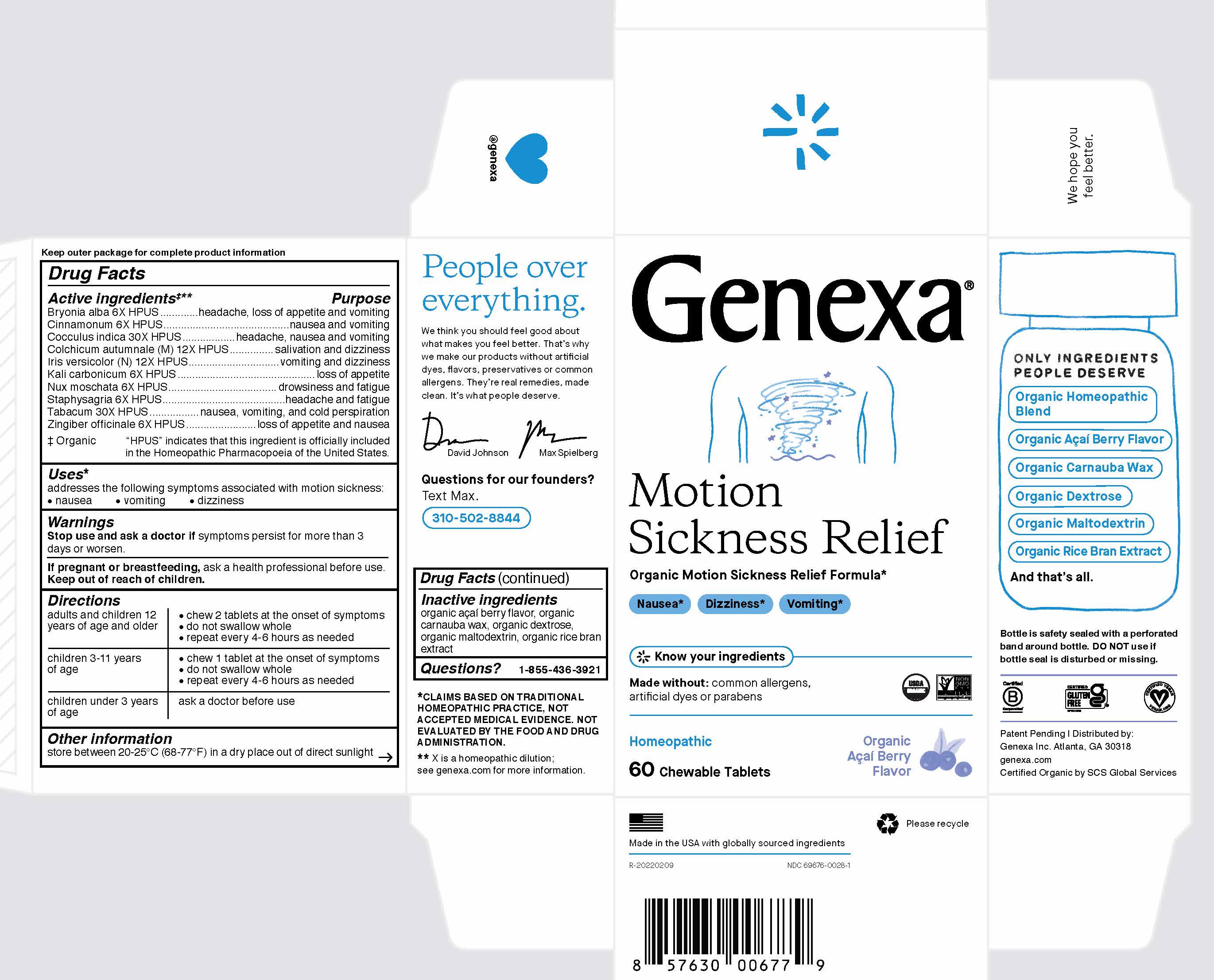
Label: GENEXA MOTION SICKNESS RELIEF- bryonia alba, cinnamonum, cocculus indica, colchicum autumnale, iris versicolor, kali carbonicum, nux moschata, staphysagria, tabacum, zingiber officinale tablet, chewable
- NDC Code(s): 69676-0028-1
- Packager: Genexa Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
ACTIVE INGREDIENT
Active ingredients ‡**
Bryonia alba 6X HPUS
Cinnamonum 6X HPUS
Cocculus indica 30X HPUS
Colchicum autumnale (M) 12X
Iris versicolor (N) 12X HPUS
Kali carbonicum 6X HPUS
Nux moschata 6X HPUS
Staphysagria 6X HPUS
Tabacum 30X HPUS
Zingiber officinale 6X HPUS
‡ Organic
"HPUS" indicates that this ingredient is officially included in the Homeopathic Pharmacopoeia of the United States.
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions
adults and children 12 years
of age and older
- chew 2 tablets at the onset of symptoms
- do not swallow whole
- repeat every 4-6 hours as needed
children 3-11 years of age - chew 1 tablet at the onset of symptoms
- do not swallow whole
- repeat every 4-6 hours as needed
children under 3 years of age ask a doctor before use - STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GENEXA MOTION SICKNESS RELIEF
bryonia alba, cinnamonum, cocculus indica, colchicum autumnale, iris versicolor, kali carbonicum, nux moschata, staphysagria, tabacum, zingiber officinale tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69676-0028 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BRYONIA ALBA WHOLE (UNII: 56K0VVT47P) (BRYONIA ALBA WHOLE - UNII:56K0VVT47P) BRYONIA ALBA WHOLE 6 [hp_X] CINNAMON (UNII: 5S29HWU6QB) (CINNAMON - UNII:5S29HWU6QB) CINNAMON 6 [hp_X] ANAMIRTA COCCULUS SEED (UNII: 810258W28U) (ANAMIRTA COCCULUS SEED - UNII:810258W28U) ANAMIRTA COCCULUS SEED 30 [hp_X] COLCHICUM AUTUMNALE BULB (UNII: 993QHL78E6) (COLCHICUM AUTUMNALE BULB - UNII:993QHL78E6) COLCHICUM AUTUMNALE BULB 12 [hp_X] IRIS VERSICOLOR ROOT (UNII: X43D4L3DQC) (IRIS VERSICOLOR ROOT - UNII:X43D4L3DQC) IRIS VERSICOLOR ROOT 12 [hp_X] POTASSIUM CARBONATE (UNII: BQN1B9B9HA) (CARBONATE ION - UNII:7UJQ5OPE7D) POTASSIUM CARBONATE 6 [hp_X] NUTMEG (UNII: AEE24M3MQ9) (NUTMEG - UNII:AEE24M3MQ9) NUTMEG 6 [hp_X] DELPHINIUM STAPHISAGRIA SEED (UNII: 00543AP1JV) (DELPHINIUM STAPHISAGRIA SEED - UNII:00543AP1JV) DELPHINIUM STAPHISAGRIA SEED 6 [hp_X] TOBACCO LEAF (UNII: 6YR2608RSU) (TOBACCO LEAF - UNII:6YR2608RSU) TOBACCO LEAF 30 [hp_X] GINGER (UNII: C5529G5JPQ) (GINGER - UNII:C5529G5JPQ) GINGER 6 [hp_X] Inactive Ingredients Ingredient Name Strength MALTODEXTRIN (UNII: 7CVR7L4A2D) RICE BRAN (UNII: R60QEP13IC) DEXTROSE (UNII: IY9XDZ35W2) CARNAUBA WAX (UNII: R12CBM0EIZ) Product Characteristics Color white (White to Off-white with specks) Score no score Shape ROUND Size 10mm Flavor BERRY Imprint Code G Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69676-0028-1 1 in 1 CARTON 01/21/2020 1 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/21/2020 Labeler - Genexa Inc. (079751024)