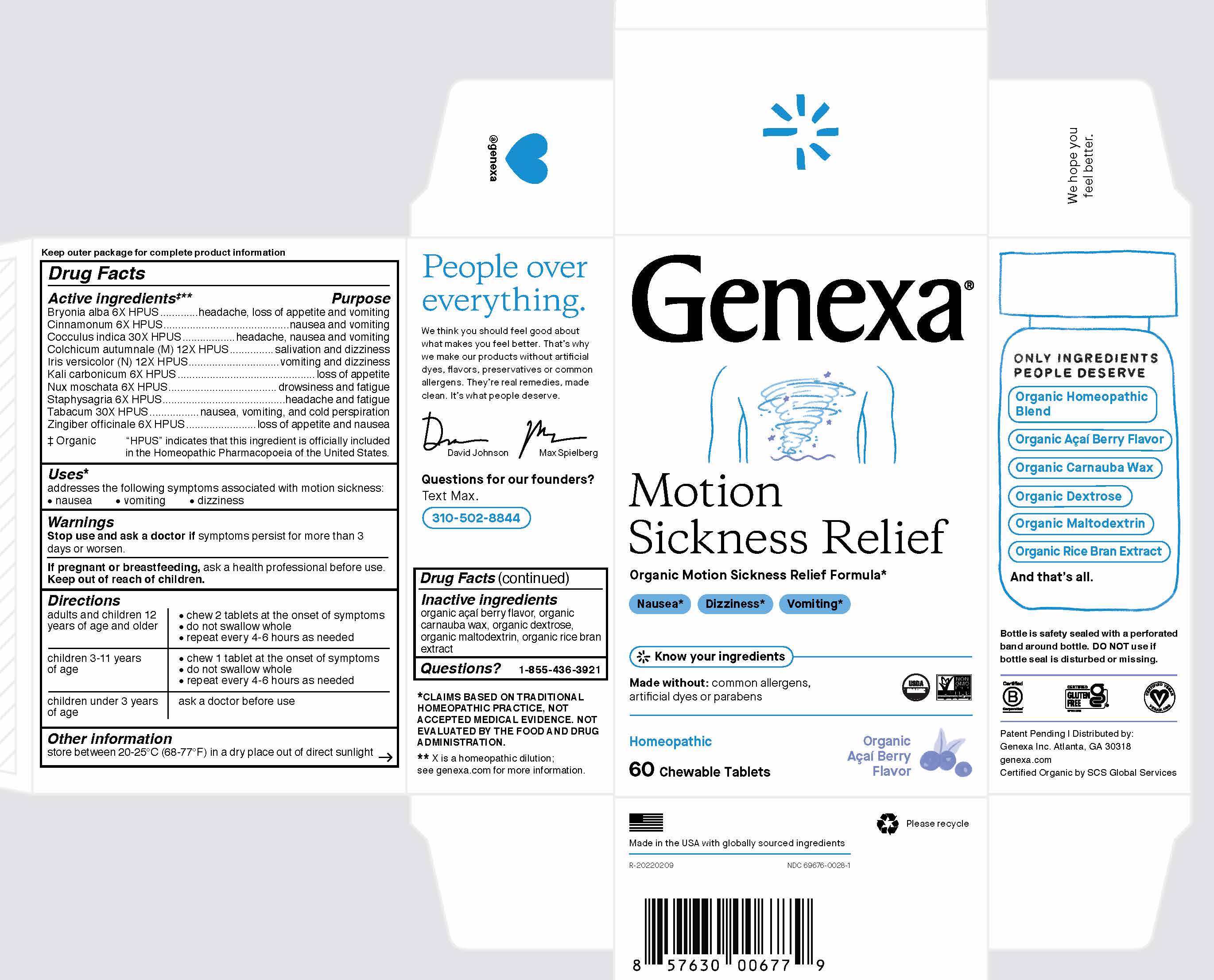
GENEXA MOTION SICKNESS RELIEF- bryonia alba, cinnamonum, cocculus indica, colchicum autumnale, iris versicolor, kali carbonicum, nux moschata, staphysagria, tabacum, zingiber officinale tablet, chewable
Genexa Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Keep outer package for complete product information
Drug Facts
Active ingredients
‡**
Bryonia alba 6X HPUS
Cinnamonum 6X HPUS
Cocculus indica 30X HPUS
Colchicum autumnale (M) 12X
Iris versicolor (N) 12X HPUS
Kali carbonicum 6X HPUS
Nux moschata 6X HPUS
Staphysagria 6X HPUS
Tabacum 30X HPUS
Zingiber officinale 6X HPUS
‡ Organic
"HPUS" indicates that this ingredient is officially included in the Homeopathic Pharmacopoeia of the United States.
Purpose
headache, loss of appetite and vomiting
nausea and vomiting
headache, nausea and vomiting
salivation and dizziness
vomiting and dizziness
loss of appetite
drowsiness and fatigue
headache and fatigue
nausea, vomiting and cold perspiration
loss of appetite and nausea
Uses*
addresses the following symptoms associated with motion sickness:
- nausea
- vomiting
- dizziness
Warnings
Stop use and ask a doctor if symptoms persist for more than 3 days or worsen.
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children.
Directions
|
adults and children 12 years
of age and older
|
- chew 2 tablets at the onset of symptoms
- do not swallow whole
- repeat every 4-6 hours as needed
|
| children 3-11 years of age |
- chew 1 tablet at the onset of symptoms
- do not swallow whole
- repeat every 4-6 hours as needed
|
| children under 3 years of age | ask a doctor before use |
Other information
store between 20-25° C (68-77° F) in a dry place out of direct sunlight.
Inactive ingredients
organic acai berry flavor, organic carnauba wax, organic dextrose, organic maltodextrin, organic rice bran extract
Questions?
1-855-436-3921
*
CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE, NOT ACCEPTED MEDICAL EVIDENCE. NOT EVALUATED BY THE FOOD AND DRUG ADMINISTRATION.
** X is a homeopathic dilution:
see genexa.com for more information.
Bottle is safety sealed with a perforated band around the bottle. DO NOT use if bottle seal is disturbed or missing.
Patent Pending | Distributed by:
Genexa Inc. Atlanta, GA 30318
genexa.com
Certified Organic by SCS Global Services
Made in the USA with globally sourced ingredients
R-20220209
NDC 69676-0028-1
Genexa
Motion Sickness Relief
Organic Motion Sickness Relief Formula*Nausea*
Nausea*
Dizziness*
Vomiting*
Know you ingredients
Made with Organic Acai Berry Flavor
Made without: common allergens, artificial dyes, or parabens
Homeopathic
60 Chewable Tablets
Genexa Inc.
