Label: BSS PLUS- balanced salt solution enriched with bicarbonate, dextrose, and glutathione kit
- NDC Code(s): 0065-0800-94
- Packager: Alcon Laboratories, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
BSS PLUS® is a sterile intraocular irrigating solution for use during all intraocular surgical procedures, including those requiring a relatively long intraocular perfusion time (e.g., pars plana vitrectomy, phacoemulsification, extra capsular cataract extraction/lens aspiration, anterior segment reconstruction, etc.). The solution does not contain a preservative and should be prepared just prior to use in surgery.
Part I: Part I is a sterile 480 mL solution in a 500 mL single-dose bag to which the Part II concentrate is added. Each mL of Part I contains: sodium chloride 7.44 mg, potassium chloride 0.395 mg, dibasic sodium phosphate 0.433 mg, sodium bicarbonate 2.19 mg, hydrochloric acid and/or sodium hydroxide (to adjust pH), in water for injection.
Part II: Part II is a sterile concentrate in a 20 mL single-dose vial for addition to Part I. Each mL of Part II contains: calcium chloride dihydrate 3.85 mg, magnesium chloride hexahydrate 5 mg, dextrose 23 mg, glutathione disulfide (oxidized glutathione) 4.6 mg, in water for injection.
After addition of BSS PLUS solution Part II to the Part I bag, each mL of the reconstituted product contains: sodium chloride 7.14 mg, potassium chloride 0.38 mg, calcium chloride dihydrate 0.154 mg, magnesium chloride hexahydrate 0.2 mg, dibasic sodium phosphate 0.42 mg, sodium bicarbonate 2.1 mg, dextrose 0.92 mg, glutathione disulfide (oxidized glutathione) 0.184 mg, hydrochloric acid and/or sodium hydroxide (to adjust pH), in water for injection.
The reconstituted product has a pH of approximately 7.4. Osmolality is approximately 305 mOsm. -
CLINICAL PHARMACOLOGY
None of the components of BSS PLUS are foreign to the eye. Human perfused cornea studies have shown BSS PLUS to be an effective irrigation solution for providing corneal detumescence and maintaining corneal endothelial integrity during intraocular perfusion. An in vivo study in rabbits has shown that BSS PLUS is more suitable than normal saline or Balanced Salt Solution for intravitreal irrigation because BSS PLUS contains the appropriate bicarbonate, pH, and ionic composition necessary for the maintenance of normal retinal electrical activity. Human in vivo studies have demonstrated BSS PLUS to be safe and effective when used during surgical procedures such as pars plana vitrectomy, phacoemulsification, cataract extraction/lens aspiration, anterior segment reconstruction. No differences have been observed between adults and pediatric patients following use of this drug product.
- INDICATIONS AND USAGE:
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
DO NOT USE BSS PLUS solution UNTIL PART I IS FULLY RECONSTITUTED WITH PART II. Discard unused contents. BSS PLUS does not contain a preservative; therefore, do not use this container for more than one patient. DISCARD ANY UNUSED PORTION SIX HOURS AFTER PREPARATION. Studies suggest that intraocular irrigating solutions which are iso-osmotic with normal aqueous fluids should be used with caution in diabetic patients undergoing vitrectomy since intraoperative lens changes have been observed.
There have been reports of corneal clouding or edema following ocular surgery in which BSS PLUS solution was used as an irrigating solution. As in all surgical procedures appropriate measures should be taken to minimize trauma to the cornea and other ocular tissues.Preparation
Reconstitute BSS PLUS® Intraocular Irrigating Solution just prior to use in surgery. Follow the same strict aseptic procedures in the reconstitution of BSS PLUS as is used for intravenous additives. Remove the blue flip-off seal from the BSS PLUS Part I (480 mL) bag. Remove the blue flip-off seal from the BSS PLUS Part II (20 mL) vial. Clean and disinfect the rubber stoppers on both containers by using sterile alcohol wipes. Transfer the contents of the Part II vial to the Part I bag using a BSS PLUS Vacuum Transfer Device (provided). An alternative method of solution transfer may be accomplished by using a 20 mL syringe to remove the Part II solution from the vial and transferring exactly 20 mL to the Part I container through the outer target area of the rubber stopper. An excess volume of Part II is provided in each vial. Gently agitate the contents to mix the solution. Place a sterile cap on the bag. Remove the tear-off portion of the label. Record the time and date of reconstitution and the patient's name on the bag label.
- GERIATRIC USE
- ADVERSE REACTIONS
-
DOSAGE AND ADMINISTRATION
The solution should be used according to the standard technique employed by the operating surgeon. Use an administration set with an air-inlet in the plastic spike since the bag does not contain a separate airway tube. Follow the directions for the particular administration set to be used. Insert the spike aseptically into the bag through the center target area of the rubber stopper. Allow the fluid to flow to remove air from the tubing before intraocular irrigation begins.
-
HOW SUPPLIED
BSS PLUS solution is supplied in two packages for reconstitution prior to use: a 500 mL bag containing 480 mL (Part I) and a 20 mL glass vial (Part II); both using grey butyl stoppers and aluminum seals. See the PRECAUTIONS section regarding reconstitution of the solution.
NDC 0065-0800-94 -
STORAGE
Store Part I and Part II at 2˚C to 25˚C (36°F to 77° F). Discard prepared solution after six hours.
Revised: April 2023
Alcon
Distributed by:
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 USA
© 2023 Alcon Inc.RECONSTITUTION INSTRUCTIONS
DIRECTIONS: Use Aseptic Technique
- Remove the BSS PLUS™ solution Part I (480 mL) bag from the clear overwrap. Remove the blue flip-off seal from the BSS PLUS solution Part II (20 mL) vial. Prepare the stoppers on both parts by using sterile alcohol wipes.
- Peel open a BSS PLUS solution Dual-Spike Transfer Device package (supplied) and remove the sterile transfer spike. NOTE: This device is vented permitting air to enter vial during solution transfer, thereby preventing the creation of a vacuum inside the vial.
- Remove protector from the white plastic piercing pin of the Dual-Spike Transfer Device.
- Firmly grasp device from behind the flange and insert the white plastic piercing pin into the upright rubber stopper of the BSS PLUS solution Part II (20 mL) vial.
- Remove guard from filter needle. Firmly grasp vial in the palm of one hand and with thumb and index finger, hold plastic flange against top of vial.
- Hold the vial in the upright position, incline and immediately insert the transfer device into the center target of rubber stopper of the BSS PLUS solution Part I (480 mL) bag. (See illustration.) NOTE: Do not invert Part II (20mL) vial before or during spiking of the BSS PLUS solution Part I bag rubber stopper, until ready to initiate transfer.
- Invert vial to initiate the transfer of Part II into Part I. If transfer does not occur immediately, gentle manipulation of the bag can initiate transfer. NOTE: An excess amount of BSS PLUS solution Part II is provided in each vial. A non-transferred solution residual of approximately 0.3 mL can be expected to remain in the vial.
- Immediately remove vial (with spike attached) from the BSS PLUS solution Part I container and discard it after solution transfer has been completed.
- Place a sterile safety cap over the rubber stopper of Part I if the solution is not going to be used immediately. Mix the solution gently until uniform. Record the patient’s name and the date and time of reconstitution. BSS PLUS solution is now ready for use.
CAUTION: Reconstituted BSS PLUS solution must be used within six hours of mixing.
Discard any solution which has aged beyond that time. Never use the same bag of BSS PLUS solution on more than one patient.
Alternative Transfer Method
If preferred, the contents of the BSS PLUS Part II component may be aspirated with an 18-gauge cannula attached to a 20 mL syringe and then transferred into the Part I bag.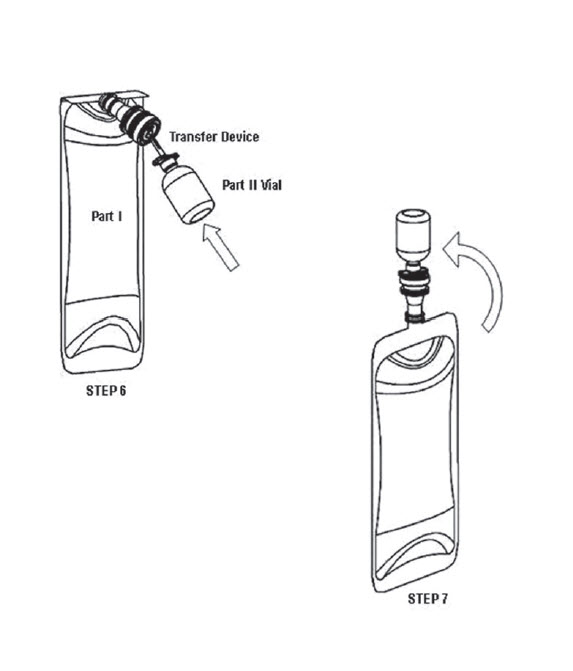
Alcon
Distributed by:
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 USA
© 2023 Alcon Inc. -
PRINCIPAL DISPLAY PANEL
NDC 0065-0800-94
BSS PLUS™ PART I 480 mL
STERILE SOLUTION
(For Reconstitution by Addition of BSS PLUS™
Concentrate Part II, 20 mL)*
SINGLE PATIENT USE ONLY
*Reconstitute just prior to use in Surgery.
BSS PLUS™ 500 mL
STERILE INTRAOCULAR IRRIGATING SOLUTION
(balanced salt solution enriched with bicarbonate, dextrose, and glutathione)
After addition of BSS PLUS – Part II (20 mL), each mL contains: sodium chloride 7.14 mg, potassium chloride 0.38 mg, calcium chloride dihydrate 0.154 mg, magnesium chloride hexahydrate 0.2 mg, dibasic sodium phosphate 0.42 mg, sodium bicarbonate 2.1 mg, dextrose 0.92 mg, glutathione disulfide (oxidized glutathione) 0.184 mg, hydrochloric acid and/or sodium hydroxide (to adjust pH), in water for injection.
pH Approx. 7.4. Osmolality Approx. 305 mOsm/kg
Rx Only
NOT FOR INJECTION OR
INTRAVENOUS INFUSION
STORAGE: Store at 36° - 77°F (2° - 25°C)
See Warnings, Precautions
and Preparation sections of
package insert.
Alcon
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 USA
LOT:
EXP.:
MFD.:
300049016-0821
Patient____________________
Reconstituted at_____________
AM/PM on_________________

NDC 0065-0800-94
BSS PLUS® PART I 480 mL
STERILE SOLUTION
(For Reconstitution by Addition of BSS PLUS®
Concentrate Part II, 20 mL)*
SINGLE PATIENT USE ONLY
*Reconstitute just prior to use in Surgery.
BSS PLUS* 500 mL
STERILE INTRAOCULAR IRRIGATING SOLUTION
(balanced salt solution enriched with bicarbonate, dextrose, and glutathione)
After addition of BSS PLUS – Part II (20 mL), each mL contains: sodium chloride 7.14 mg, potassium chloride 0.38 mg, calcium chloride dihydrate 0.154 mg, magnesium chloride hexahydrate 0.2 mg, dibasic sodium phosphate 0.42 mg, sodium bicarbonate 2.1 mg, dextrose 0.92 mg, glutathione disulfide (oxidized glutathione) 0.184 mg, hydrochloric acid and/or sodium hydroxide (to adjust pH), in water for injection.
pH Approx. 7.4. Osmolality Approx. 305 mOsm/kg
Rx Only
NOT FOR INJECTION OR
INTRAVENOUS INFUSION
STORAGE: Store at 36° - 77°F (2° - 25°C)
See Warnings, Precautions
and Preparation sections of
package insert.
Alcon®
a Novartis company
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 USA
© 2013, 2015 Novartis
LOT:
EXP.:
MFD.:
9012637-1115
Patient____________________
Reconstituted at_____________
AM/PM on_________________

NDC 0065-0800-94 20mL
BSS PLUS™
CONCENTRATE PART II
(Sterile Solution for Reconstitution)
Alcon
Alcon Laboratories Inc.
Fort Worth, Texas 76134 USA
Each mL contains: calcium chloride dihydrate 3.85 mg, magnesium chloride hexahydrate 5 mg, dextrose 23 mg, glutathione disulfide (oxidized glutathione) 4.6 mg, in water for injection.
For Addition to BSS PLUS™ — Part I (480 mL) only.
See Preparation section of package insert.
WARNINGS: Not for injection or intravenous infusion – for reconstitution of irrigation solution only. Do not use unless product is clear, seal is intact and container is undamaged. Do not use if product is discolored or contains a precipitate.
PRECAUTIONS: Discard unused contents. Do not use this container for more than one patient.
Storage: Store at 2-25°C (36-77°F).
Rx Only
300049017-0621
LOT:
EXP.:
MFD.:

NDC 0065-0800-94 20mL
BSS PLUS®
CONCENTRATE PART II
(Sterile Solution for Reconstitution)
Alcon®
Alcon Laboratories Inc.
Fort Worth, Texas 76134 USA
Each mL contains: calcium chloride dihydrate 3.85 mg, magnesium chloride hexahydrate 5 mg, dextrose 23 mg, glutathione disulfide (oxidized glutathione) 4.6 mg, in water for injection.
For Addition to BSS PLUS® — Part I (480 mL) only.
See Preparation section of package insert.
WARNINGS: Not for injection or intravenous infusion – for reconstitution of irrigation solution only. Do not use unless product is clear, seal is intact and container is undamaged. Do not use if product is discolored or contains a precipitate.
PRECAUTIONS: Discard unused contents. Do not use this container for more than one patient.
Storage: Store at 2-25°C (36-77°F).
Rx Only
© 2002-2003, 2013, 2015 Novartis
H14141-1115
LOT:
EXP.:
MFD.:

BSS PLUS™
STERILE INTRAOCULAR
IRRIGATING SOLUTION
(balanced salt solution enriched with bicarbonate, dextrose, and glutathione)
1 Vial BSS PLUS® Concentrate Part II Sterile Solution for Reconstitution with BSS PLUS solution Part 1 Only
1 BSS PLUS solution Dual Spike Transfer Device
1 BSS PLUS solution Product Package Insert
WARNINGS: For IRRIGATION during ophthalmic surgery only. Not for injection or intravenous infusion. Do not use unless product is clear, seal is intact and container is undamaged. Do not use if product is discolored or contains a precipitate.
Store at 2° - 25°C (36° - 77°F).
Alcon
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 USA
300049018-0621
PRECAUTIONS: DO NOT USE BSS PLUS* Sterile Intraocular Irrigating Solution, UNTIL PART I IS FULLY RECONSTITUTED WITH PART II. Discard unused contents. BSS PLUS solution does not contain a preservative; therefore, do not use this container for more than one patient. DISCARD ANY UNUSED PORTION SIX HOURS AFTER PREPARATION. Studies suggest that intraocular irrigating solutions which are iso-osmotic with normal aqueous fluids should be used with caution in diabetic patients undergoing vitrectomy since intraoperative lens changes have been observed.
There have been reports of corneal clouding or edema following ocular surgery in which BSS PLUS solution was used as irrigating solution. As in all surgical procedures, appropriate measures should be taken to minimize trauma to the cornea and other ocular tissues.
LOT:
EXP:
MFD:

BSS PLUS®
STERILE INTRAOCULAR
IRRIGATING SOLUTION
(balanced salt solution enriched with bicarbonate, dextrose, and glutathione)
1 Vial BSS PLUS® Concentrate Part II Sterile Solution for Reconstitution with BSS PLUS solution Part 1 Only
1 BSS PLUS solution Dual Spike Transfer Device
1 BSS PLUS solution Product Package Insert
WARNINGS: For IRRIGATION during ophthalmic surgery only. Not for injection or intravenous infusion. Do not use unless product is clear, seal is intact and container is undamaged. Do not use if product is discolored or contains a precipitate.
Store at 2° - 25°C (36° - 77°F).
Alcon®
a Novartis company
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 USA
© 2013, 2014, 2015 Novartis
9012757-0116
PRECAUTIONS: DO NOT USE BSS PLUS* Sterile Intraocular Irrigating Solution, UNTIL PART I IS FULLY RECONSTITUTED WITH PART II. Discard unused contents. BSS PLUS solution does not contain a preservative; therefore, do not use this container for more than one patient. DISCARD ANY UNUSED PORTION SIX HOURS AFTER PREPARATION. Studies suggest that intraocular irrigating solutions which are iso-osmotic with normal aqueous fluids should be used with caution in diabetic patients undergoing vitrectomy since intraoperative lens changes have been observed.
There have been reports of corneal clouding or edema following ocular surgery in which BSS PLUS solution was used as irrigating solution. As in all surgical procedures, appropriate measures should be taken to minimize trauma to the cornea and other ocular tissues.
LOT:
EXP:
MFD:

-
INGREDIENTS AND APPEARANCE
BSS PLUS
balanced salt solution enriched with bicarbonate, dextrose, and glutathione kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0065-0800 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0065-0800-94 1 in 1 PACKAGE; Type 0: Not a Combination Product 06/20/2013 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BAG 480 mL Part 2 1 VIAL, GLASS 20 mL Part 1 of 2 PART 1
balanced salt solution enriched with bicarbonate, dextrose, and glutathione solutionProduct Information Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium Chloride (UNII: 451W47IQ8X) (Sodium Cation - UNII:LYR4M0NH37, Chloride Ion - UNII:Q32ZN48698) Sodium Chloride 7.44 mg in 1 mL Potassium Chloride (UNII: 660YQ98I10) (Potassium Cation - UNII:295O53K152, Chloride Ion - UNII:Q32ZN48698) Potassium Chloride 0.395 mg in 1 mL Sodium Phosphate, Dibasic (UNII: GR686LBA74) (Sodium Cation - UNII:LYR4M0NH37, Phosphate Ion - UNII:NK08V8K8HR) Sodium Phosphate, Dibasic 0.433 mg in 1 mL Sodium Bicarbonate (UNII: 8MDF5V39QO) (Sodium Cation - UNII:LYR4M0NH37, Bicarbonate Ion - UNII:HN1ZRA3Q20) Sodium Bicarbonate 2.19 mg in 1 mL Inactive Ingredients Ingredient Name Strength Hydrochloric Acid (UNII: QTT17582CB) Sodium Hydroxide (UNII: 55X04QC32I) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 480 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018469 06/20/2013 Part 2 of 2 PART II
calcium chloride, magnesium chloride, dextrose, and glutathione concentrateProduct Information Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Calcium Chloride (UNII: M4I0D6VV5M) (Calcium Cation - UNII:2M83C4R6ZB, Chloride Ion - UNII:Q32ZN48698) Calcium Chloride 3.85 mg in 1 mL Magnesium Chloride (UNII: 02F3473H9O) (Magnesium Cation - UNII:T6V3LHY838, Chloride Ion - UNII:Q32ZN48698) Magnesium Chloride 5 mg in 1 mL Dextrose, Unspecified Form (UNII: IY9XDZ35W2) (Anhydrous Dextrose - UNII:5SL0G7R0OK) Dextrose, Unspecified Form 23 mg in 1 mL Oxiglutatione (UNII: ULW86O013H) (Oxiglutatione - UNII:ULW86O013H) Oxiglutatione 4.6 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 20 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018469 06/20/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018469 06/20/2013 Labeler - Alcon Laboratories, Inc. (008018525) Registrant - Alcon Laboratories, Inc. (008018525) Establishment Name Address ID/FEI Business Operations Alcon Research LLC 007672236 manufacture(0065-0800)






