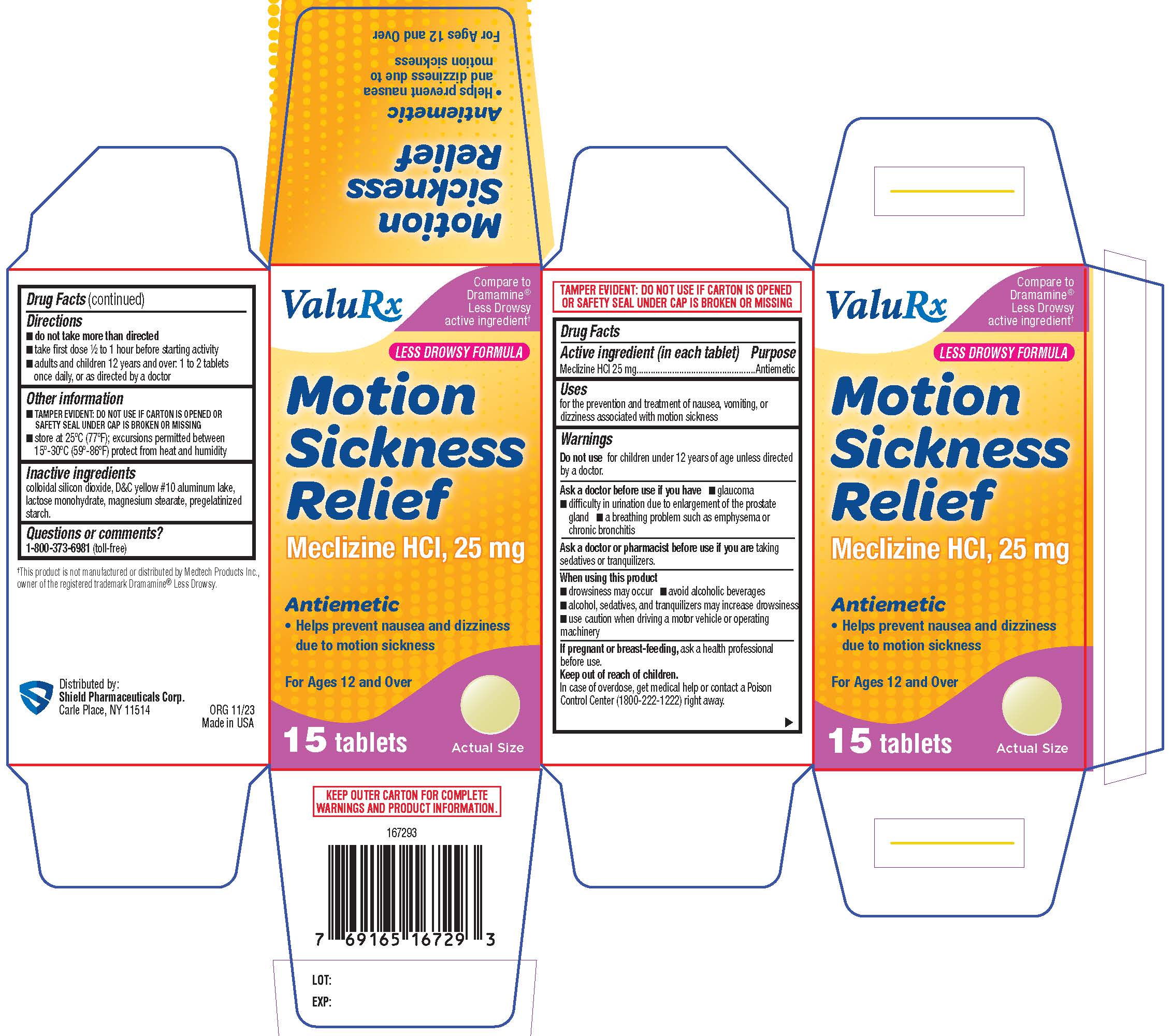
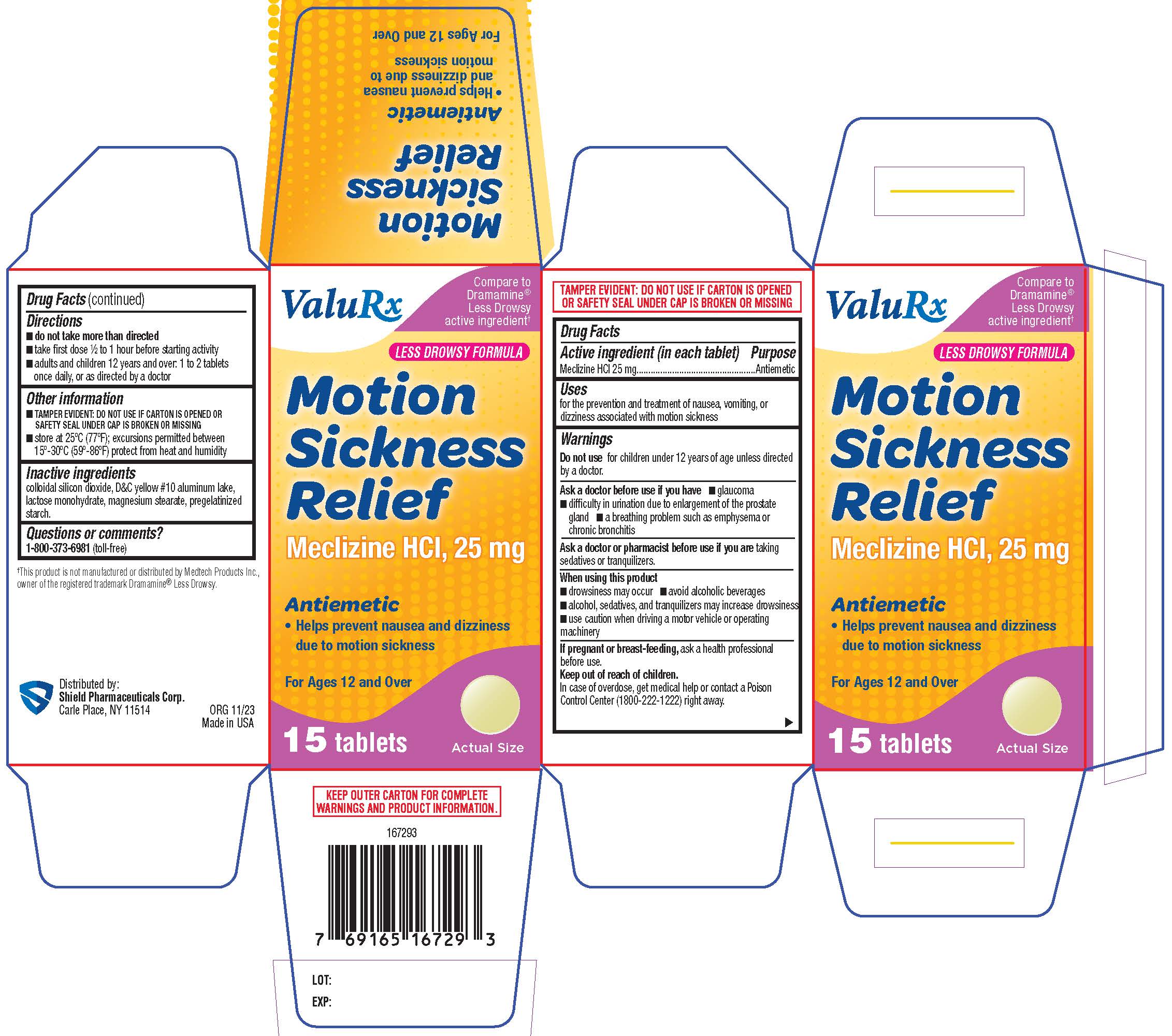
Label: MOTION SICKNESS RELIEF- meclizine hcl tablet, film coated
- NDC Code(s): 83059-0007-1
- Packager: Shield Pharmaceuticals Corp
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each liquid-filled capsule)
- Purpose
- Uses
- Warnings
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use if you are
- When using this product
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MOTION SICKNESS RELIEF
meclizine hcl tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83059-0007 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MECLIZINE HYDROCHLORIDE (UNII: HDP7W44CIO) (MECLIZINE - UNII:3L5TQ84570) MECLIZINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) D&C YELLOW NO. 10 ALUMINUM LAKE (UNII: CQ3XH3DET6) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color yellow Score no score Shape ROUND Size 8mm Flavor Imprint Code 44;403 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83059-0007-1 1 in 1 PACKAGE 12/06/2023 1 15 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M002 12/06/2023 Labeler - Shield Pharmaceuticals Corp (118724924)