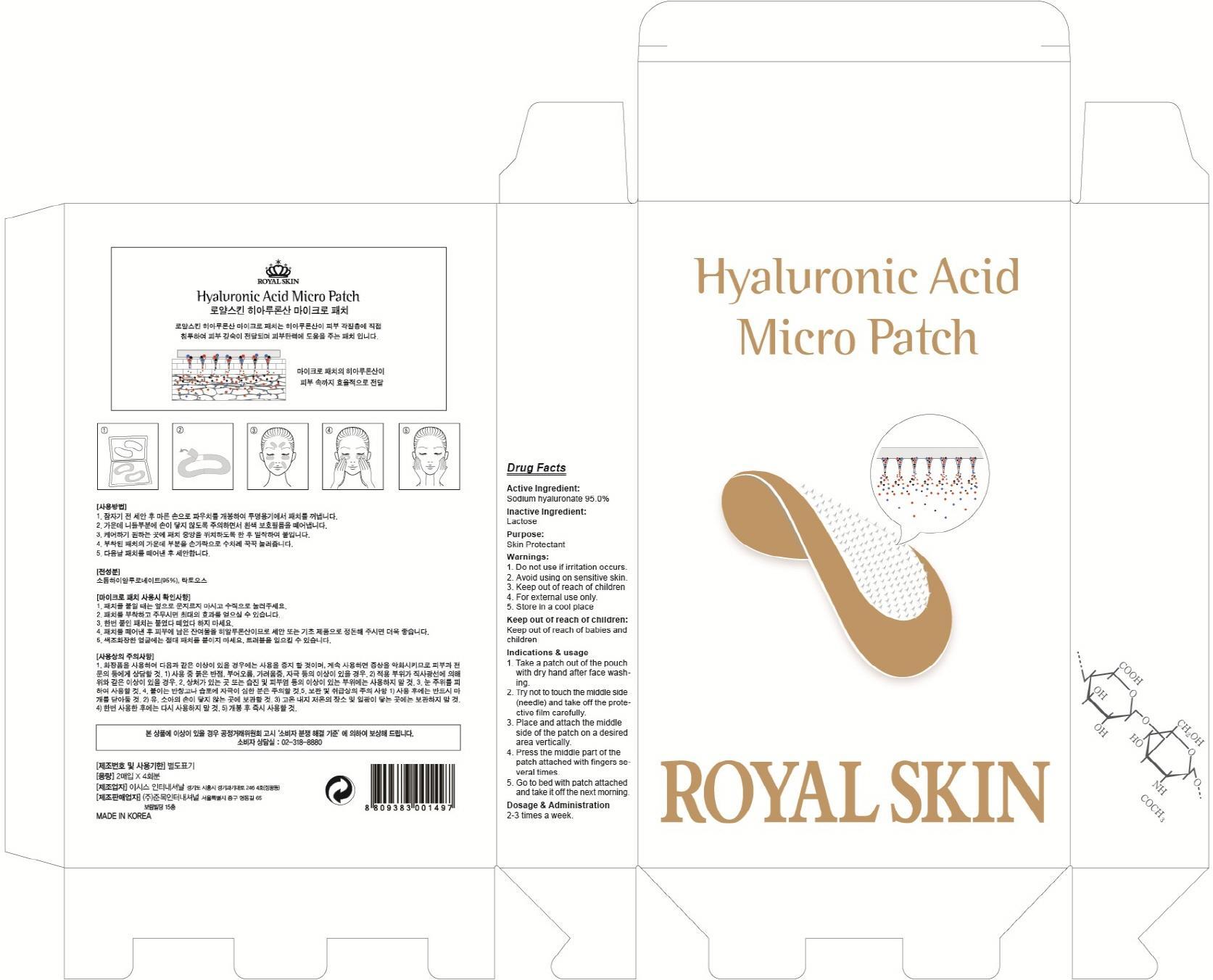
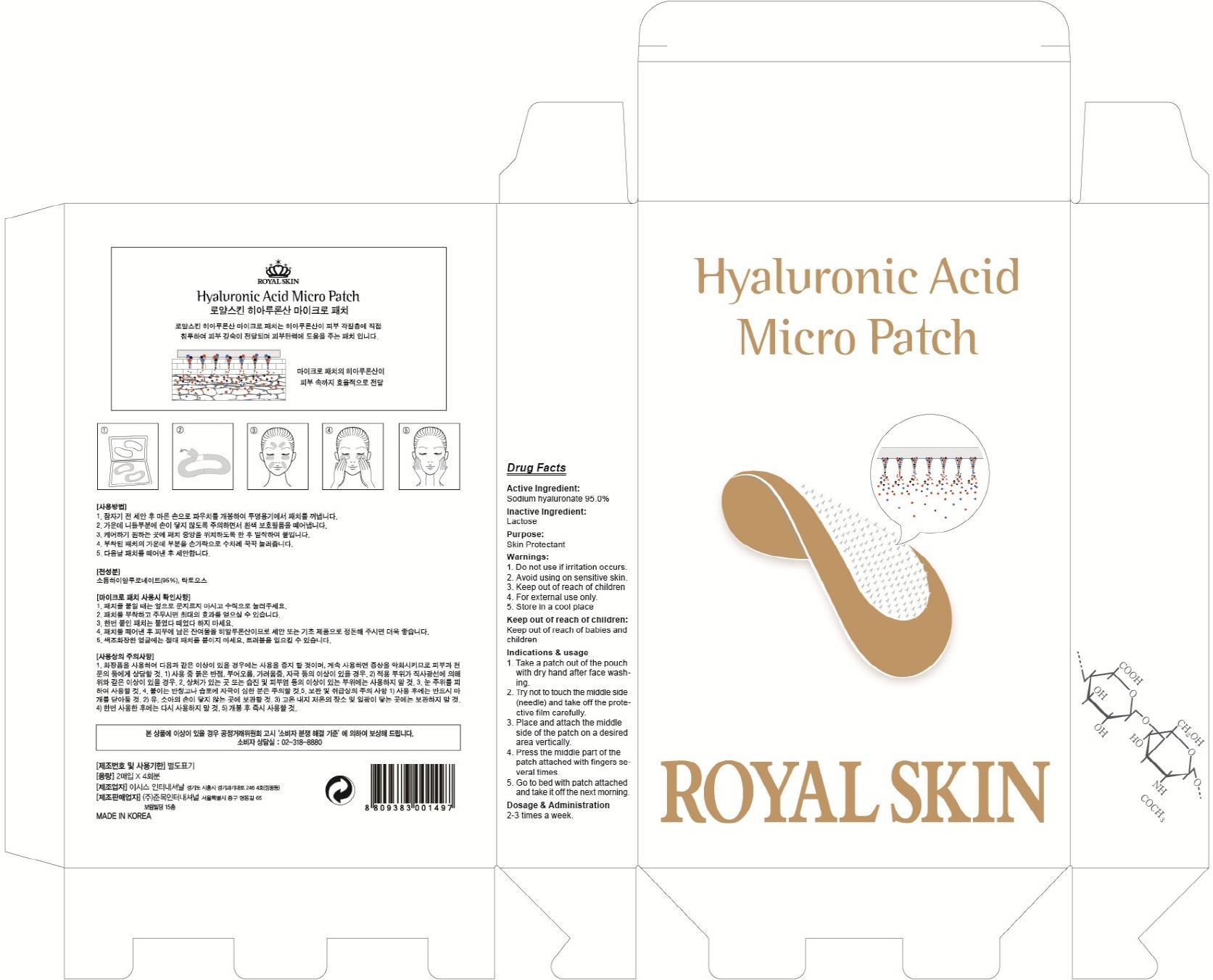
Label: HYALURONIC ACID MICRO- hyaluronate sodium patch
-
Contains inactivated NDC Code(s)
NDC Code(s): 70018-010-01 - Packager: JUNMOK INTERNATIONAL,INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated August 6, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
INDICATIONS & USAGE
Indications & usage:1. Take a patch out of the pouch with dry hand after face washing. 2. Try not to touch the middle side (needle) and take off the protective film carefully. 3. Place and attach the middle side of the patch on a desired area vertically. 4. Press the middle part of the patch attached with fingers several times. 5. Go to bed with patch attached and take it off the next morning.
- DOSAGE & ADMINISTRATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HYALURONIC ACID MICRO
hyaluronate sodium patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70018-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYALURONATE SODIUM (UNII: YSE9PPT4TH) (HYALURONIC ACID - UNII:S270N0TRQY) HYALURONATE SODIUM 1.14 g in 4 Inactive Ingredients Ingredient Name Strength Lactose (UNII: J2B2A4N98G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70018-010-01 4 in 1 CARTON; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/01/2015 Labeler - JUNMOK INTERNATIONAL,INC (689045829) Registrant - JUNMOK INTERNATIONAL,INC (689045829) Establishment Name Address ID/FEI Business Operations JUNMOK INTERNATIONAL,INC 689045829 manufacture(70018-010)