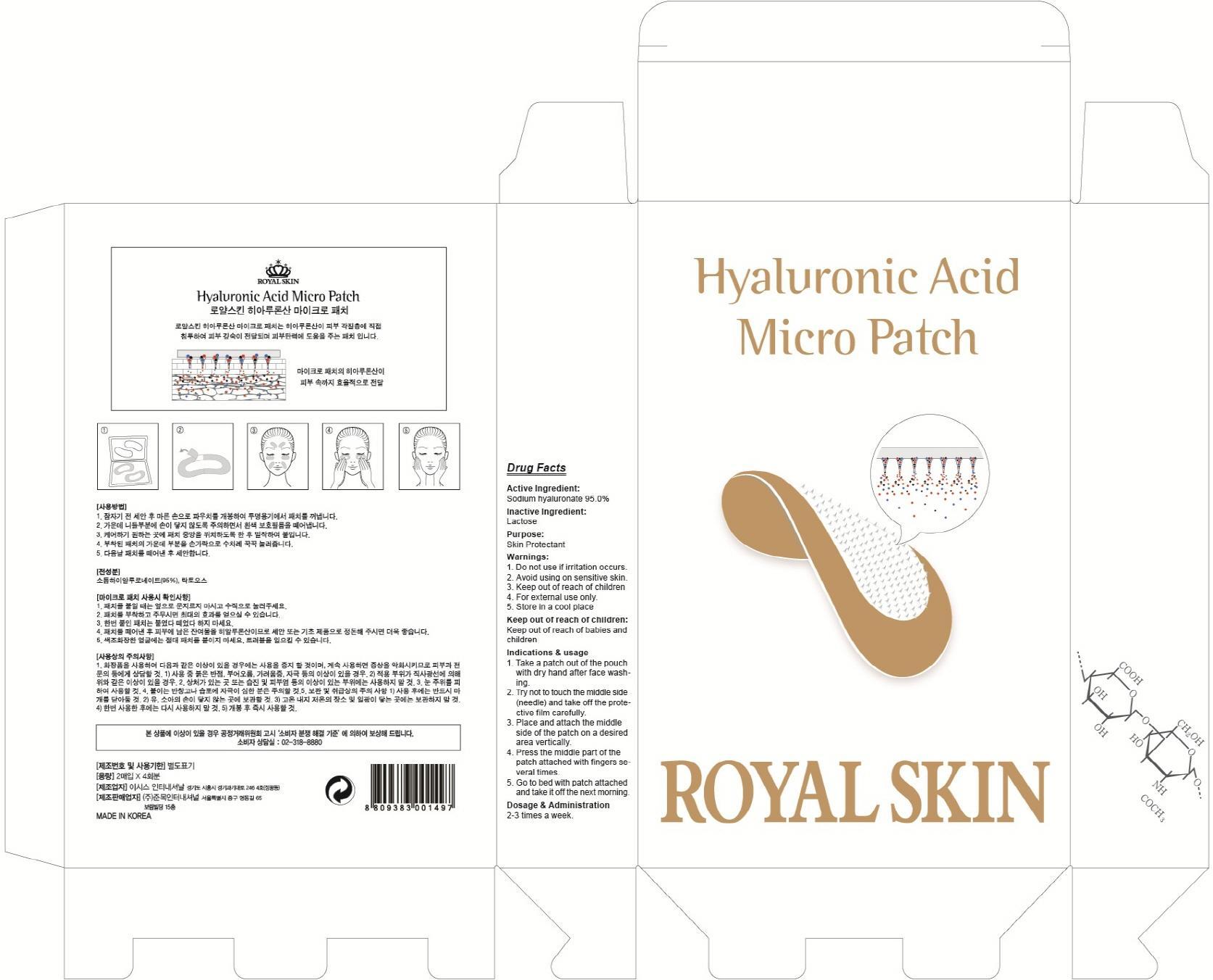
WARNINGS
Warnings: 1. Do not use if irritation occurs. 2. Avoid using on sensitive skin. 3. Keep out of reach of children 4. For external use only. 5. Store in a cool place
INDICATIONS & USAGE
Indications & usage:1. Take a patch out of the pouch with dry hand after face washing. 2. Try not to touch the middle side (needle) and take off the protective film carefully. 3. Place and attach the middle side of the patch on a desired area vertically. 4. Press the middle part of the patch attached with fingers several times. 5. Go to bed with patch attached and take it off the next morning.