Label: CLOSYS SILVER SENSITIVE MOUTH RINSE FLUORIDE RINSE- sodium fluoride rinse
- NDC Code(s): 58578-0517-1
- Packager: Rowpar Pharmaceuticals, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
-
Directions
Adults and children 6 years of age and older
- use twice a day after brushing your teeth with a toothpaste
- pour out and vigorously swish 10 milliliters of rinse between your teeth for 1 minute and then spit out
- do not swallow the rinse
- do not eat or drink for 30 minutes after rinsing
- instruct children under 12 years of age in good rinsing habits (to minimize swallowing)
- supervise children as necessary until capable of using without supervision
Children under 6 years
- consult a dentist or doctor
- Other Information
- Inactive ingredients
- QUESTIONS
-
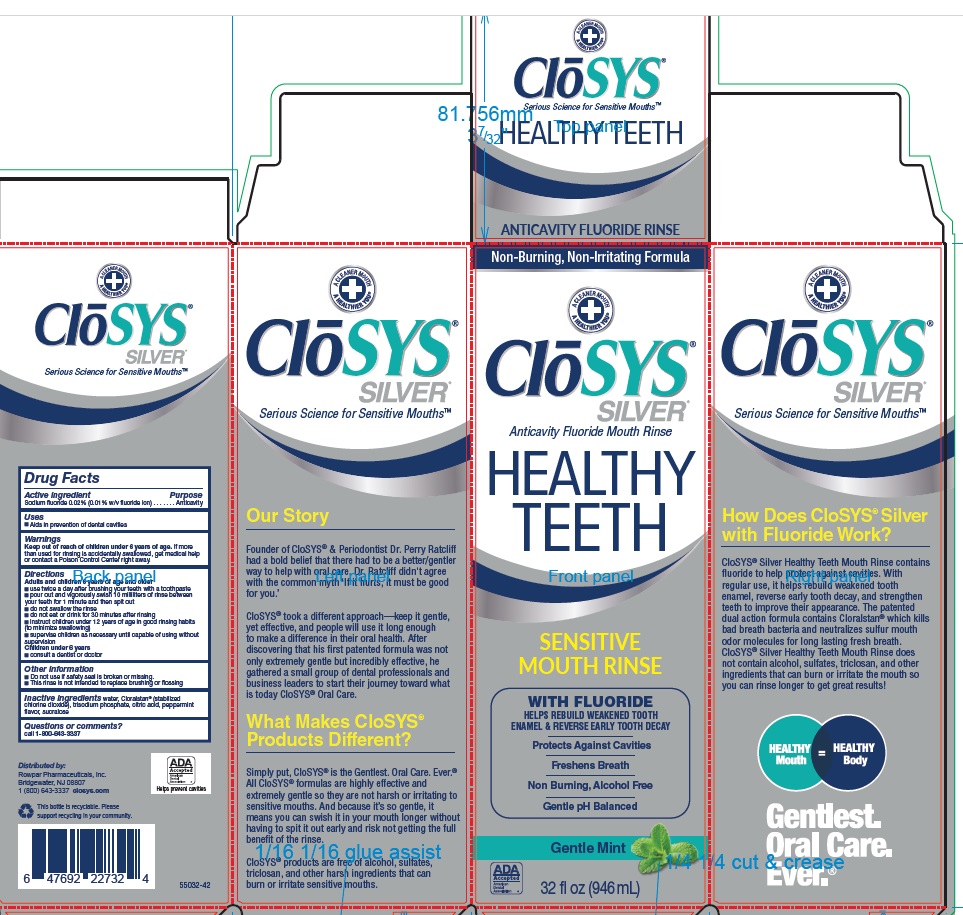
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
ClōSYS®
SILVER®
Anticavity Fluoride Mouth Rinse
HEALTHY TEETH
SENSITIVE MOUTH RINSE
WITH FLUORIDE
HELPS REBUILD WEAKENED TOOTH
ENAMEL & REVERSE EARLY TOOTH DECAY
Protects Against Cavities
Freshens Breath
Non Burning, Alcohol Free
Gentle pH Balanced
Gentle Mint
32 fl oz (946 mL) 52532-32
Distributed by:
Rowpar Pharmaceuticals, Inc.
Bridgewater, NJ 08807
1 (800) 643-3337 closys.com
This bottle is recyclable. Please
support recycling in your community 52532-33

Non-Burning, Non-Irritating Formula
A CLEANER MOUTH
A HEALTHIER YOU®
ClōSYS®
SILVER®
Anticavity Fluoride Mouth Rinse
HEALTHY
TEETH
SENSITIVE
MOUTH RINCE
WITH FLUORIDE
HELPS REBUILD WEAKENED TOOTH
ENAMEL & REVERSE EARLY TOOTH DECAY
Protects Against Cavities
Freshens Breath
Non Burning, Alcohol Free
Gentle pH Balanced
Gentle Mint
32 fl oz (946 mL)
A CLEANER MOUTH
A HEALTHIER YOU®
ClōSYS®
SILVER®
Serious Science for Sensitive Mouths™
How does CloSYS® Silver
with Flouride Work?
CloSYS® Silver Healthy Teeth Mouth Rinse contains fluoride to help protect against cavities. With regular use, it helps rebuild weakened tooth enamel, reverse early tooth decay, and strengthen teeth to improve their appearance. The patented dual action formula contains Cloralstan® which kills bad breath bacteria and neutralizes sulfur mouth odor molecules for long lasting fresh breath. CloSYS® Silver Healthy Teeth Mouth Rinse does not contain alcohol, sulfates, triclosan, and other ingredients that can burn or irritate the mouth so you can rinse longer to get great results!
HEALTHY Mouth = HEALTHY Body
Gentlest.
Oral Care.
Ever.®A CLEANER MOUTH
A HEALTHIER YOU®
ClōSYS®
SILVER®
Serious Science for Sensitive Mouths™
Our Story
Founder of CloSYS® & Periodontist Dr. Perry Ratcliff had a bold belief that there had to be a better/gentler way to help with oral care. Dr. Ratcliff didn't agree with the common myth ‘If it hurts, it must be good for you’
CloSYS® took a different approach—keep it gentle, yet effective, and people will use it long enough to make a difference in their oral health. After discovering that his first patented formula was not only extremely gentle but incredibly effective, he gathered a small group of dental professionals and business leaders to start their journey toward what is today CloSYS® Oral Care.
What Makes CloSYS®
Product Different?
Simply put, CloSYS® is the Gentlest. Oral Care. Ever.® All CloSYS® formulas are highly effective and extremely gentle so they are not harsh or irritating to sensitive mouths. And because it’s so gentle, it means you can swish it in your mouth longer without having to spit it out early and risk not getting the full benefit of the rinse.
CloSYS® products are free of alcohol, sulfates, triclosan, and other harsh ingredients that can burn or irritate sensitive mouths.Distributed by:
Rowpar Pharmaceuticals, Inc.
Bridgewater, NJ 08807
1 (800) 643-3337 closys.com
This bottle is recyclable. Please
support recycling in your community
55032-42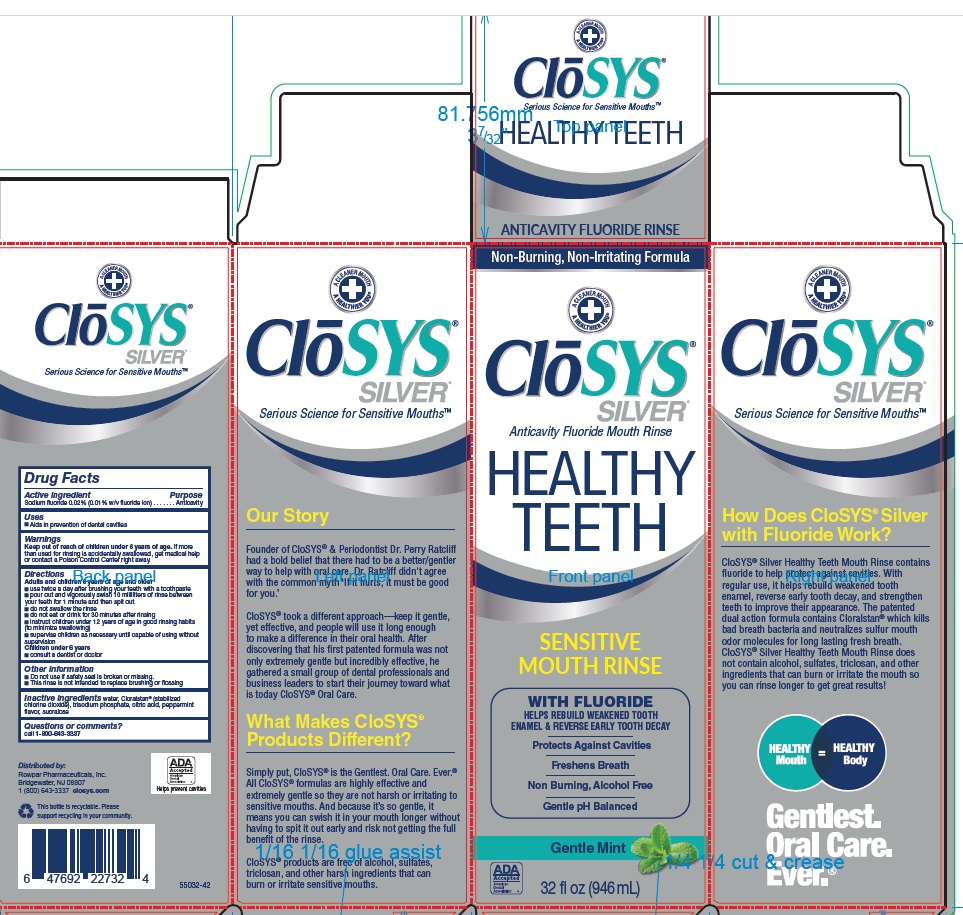
-
INGREDIENTS AND APPEARANCE
CLOSYS SILVER SENSITIVE MOUTH RINSE FLUORIDE RINSE
sodium fluoride rinseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58578-0517 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.02 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CHLORINE DIOXIDE (UNII: 8061YMS4RM) SODIUM PHOSPHATE, TRIBASIC, DODECAHYDRATE (UNII: B70850QPHR) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) PEPPERMINT (UNII: V95R5KMY2B) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color Score Shape Size Flavor PEPPERMINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58578-0517-1 1 in 1 BOX 01/01/2024 1 473 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 01/01/2023 Labeler - Rowpar Pharmaceuticals, Inc. (783704661)