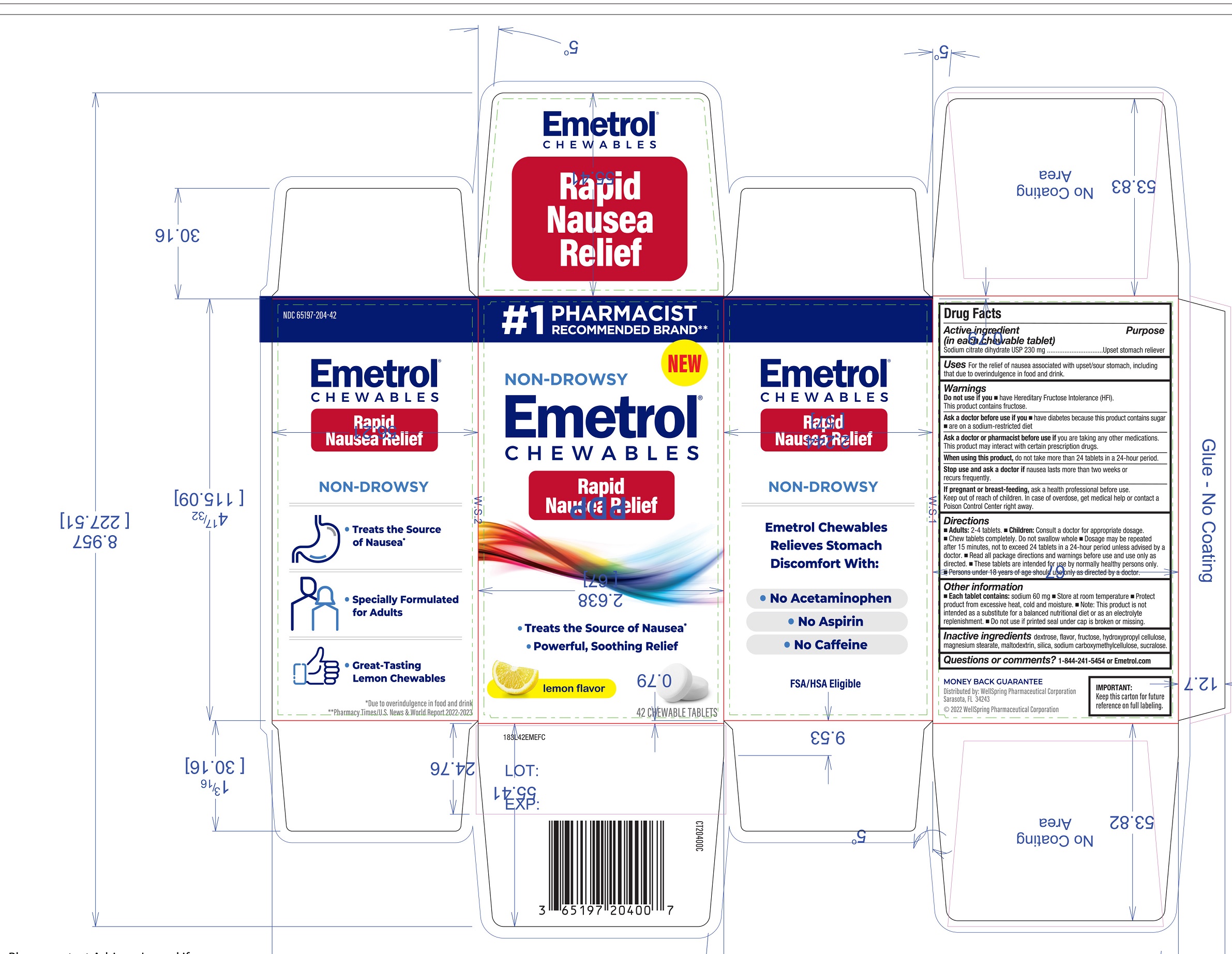
Label: EMETROL CHEWABLES- sodium citrate dihydrate tablet, chewable
- NDC Code(s): 65197-204-42
- Packager: WellSpring Pharmaceutical Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you
- •
- have diabetes because this product contains sugar
- •
- are on a sodium-restricted diet
Ask a doctor or pharmacist before use if
- •
- you are taking any other medications. This product may interact with certain prescription drugs.
-
Directions
- •
- Adults: 2-4 tablets.
- •
- Children: Consult a doctor for appropriate dosage.
- •
- Chew tablets completely. Do not swallow whole
- •
- Dosage may be repeated after 15 minutes, not to exceed 24 tablets in a 24-hour period unless advised by a doctor.
- •
- Read all package directions and warnings before use and use only as directed.
- •
- These tablets are intended for use by normally healthy persons only.
- •
- Persons under 18 years of age should use only as directed by a doctor.
-
Other information
- •
- Each table contains: Sodium 60mg
- •
- Store at room temperature
- •
- Protect product from excessive heat, cold and moisture
- •
- Note: This product is not intended as a substitute for a balanced nutritional diet or as an electrolyte replenishment.
- •
- Do not use if printed seal under cap is broken or missing.
- Inactive ingredients
- Questions or Comments?
- Distributed By
- PACKAGE LABEL
-
INGREDIENTS AND APPEARANCE
EMETROL CHEWABLES
sodium citrate dihydrate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65197-204 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 230 mg Inactive Ingredients Ingredient Name Strength DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) LEMON (UNII: 24RS0A988O) FRUCTOSE (UNII: 6YSS42VSEV) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color white (White) Score no score Shape ROUND Size 16mm Flavor LEMON Imprint Code CB2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65197-204-42 1 in 1 BOX 03/15/2022 1 42 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 03/15/2022 Labeler - WellSpring Pharmaceutical Corporation (110999054)