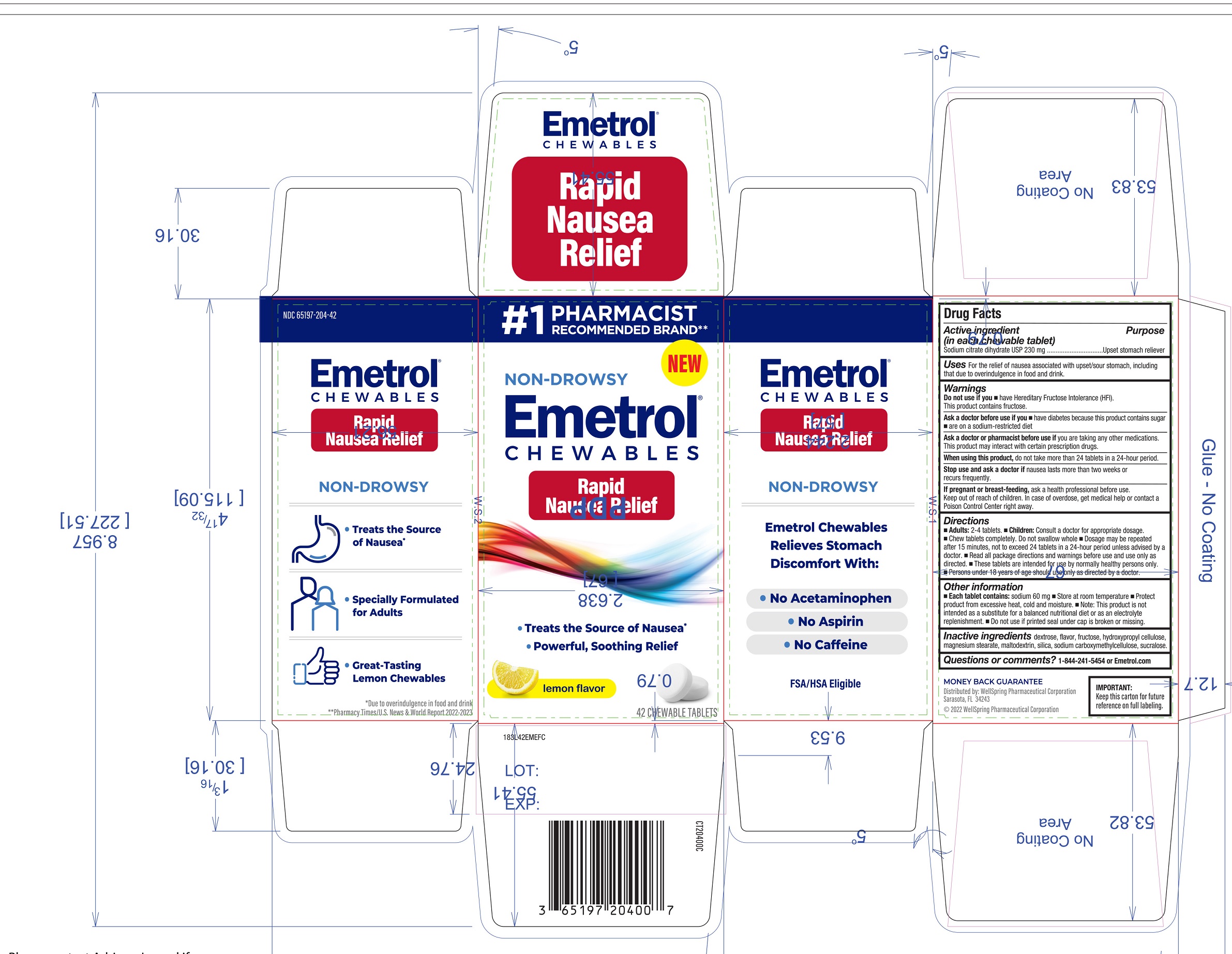
Uses
For the relief of nausea associated with upset/sour stomach, including that due to overindulgence in food and drink.
Warnings
Ask a doctor before use if you
- •
- have diabetes because this product contains sugar
- •
- are on a sodium-restricted diet
Ask a doctor or pharmacist before use if
- •
- you are taking any other medications. This product may interact with certain prescription drugs.
Directions
- •
- Adults: 2-4 tablets.
- •
- Children: Consult a doctor for appropriate dosage.
- •
- Chew tablets completely. Do not swallow whole
- •
- Dosage may be repeated after 15 minutes, not to exceed 24 tablets in a 24-hour period unless advised by a doctor.
- •
- Read all package directions and warnings before use and use only as directed.
- •
- These tablets are intended for use by normally healthy persons only.
- •
- Persons under 18 years of age should use only as directed by a doctor.
Other information
- •
- Each table contains: Sodium 60mg
- •
- Store at room temperature
- •
- Protect product from excessive heat, cold and moisture
- •
- Note: This product is not intended as a substitute for a balanced nutritional diet or as an electrolyte replenishment.
- •
- Do not use if printed seal under cap is broken or missing.
Inactive ingredients
dextrose, flavor, fructose, hydroxypropyl cellulose, magnesium stearate, maltodextrin, silica, sodium carboxymethylcellulose, sucralose.
Distributed By
WellSpring Pharmaceutical Corporation
Sarasota, FL 34243
(c) 2022 WellSpring Pharmaceutical Corporation