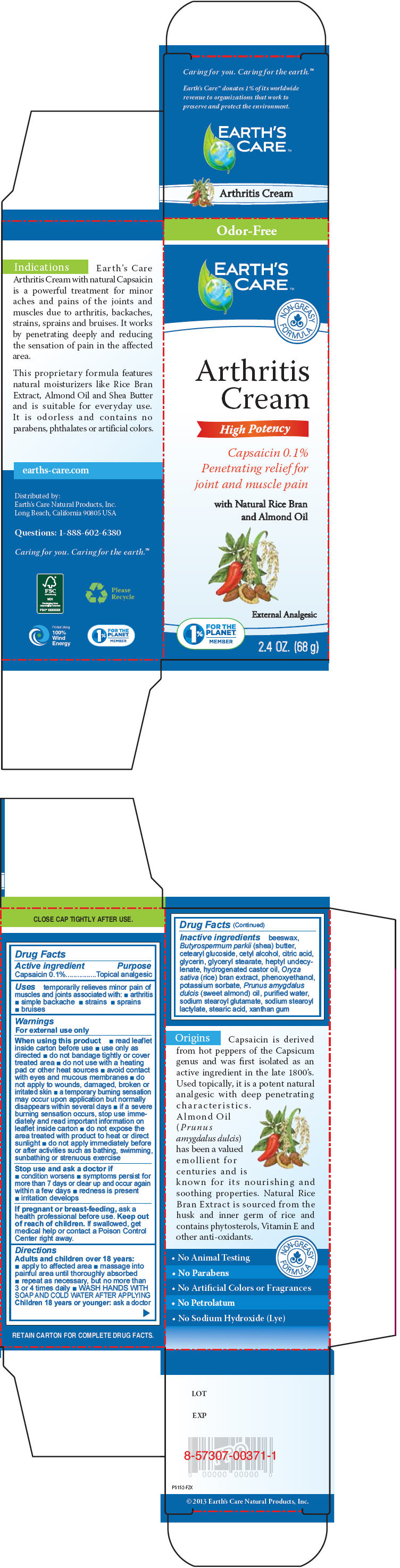
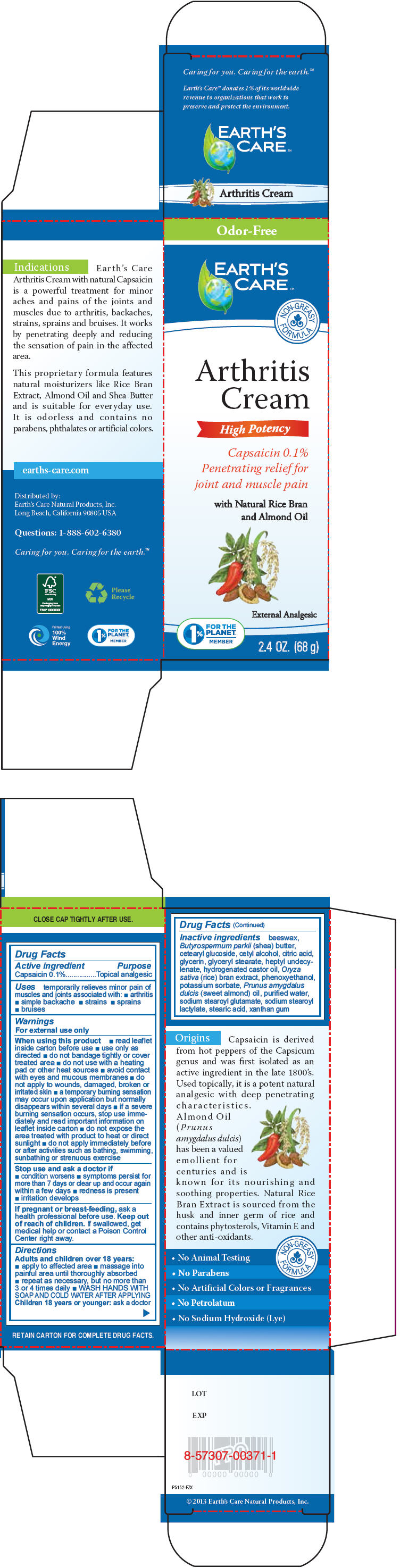
Label: EARTHS CARE ARTHRITIS- capsaicin cream
- NDC Code(s): 24286-1559-2
- Packager: DLC Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 13, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
When using this product
- read leaflet inside carton before use
- use only as directed
- do not bandage tightly or cover treated area
- do not use with a heating pad or other heat sources
- avoid contact with eyes and mucous membranes
- do not apply to wounds, damaged, broken or irritated skin
- a temporary burning sensation may occur upon application but normally disappears within several days
- if a severe burning sensation occurs, stop use immediately and read important information on leaflet inside carton
- do not expose the area treated with product to heat or direct sunlight
- do not apply immediately before or after activities such as bathing, swimming, sunbathing or strenuous exercise
- Directions
-
Inactive ingredients
beeswax, Butyrospermum parkii (shea) butter, cetearyl glucoside, cetyl alcohol, citric acid, glycerin, glyceryl stearate, heptyl undecylenate, hydrogenated castor oil, Oryza sativa (rice) bran extract, phenoxyethanol, potassium sorbate, Prunus amygdalus dulcis (sweet almond) oil, purified water, sodium stearoyl glutamate, sodium stearoyl lactylate, stearic acid, xanthan gum
- Questions
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 68 g Tube Box
-
INGREDIENTS AND APPEARANCE
EARTHS CARE ARTHRITIS
capsaicin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:24286-1559 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.1 g in 100 g Inactive Ingredients Ingredient Name Strength YELLOW WAX (UNII: 2ZA36H0S2V) SHEA BUTTER (UNII: K49155WL9Y) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) CETYL ALCOHOL (UNII: 936JST6JCN) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) HEPTYL UNDECYLENATE (UNII: W77QUB6GXO) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) RICE BRAN (UNII: R60QEP13IC) PHENOXYETHANOL (UNII: HIE492ZZ3T) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ALMOND OIL (UNII: 66YXD4DKO9) WATER (UNII: 059QF0KO0R) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) SODIUM STEAROYL LACTYLATE (UNII: IN99IT31LN) STEARIC ACID (UNII: 4ELV7Z65AP) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24286-1559-2 1 in 1 BOX 09/25/2013 1 68 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part348 09/25/2013 Labeler - DLC Laboratories, Inc. (093351930) Establishment Name Address ID/FEI Business Operations DLC Laboratories, Inc. 093351930 MANUFACTURE(24286-1559) , LABEL(24286-1559)