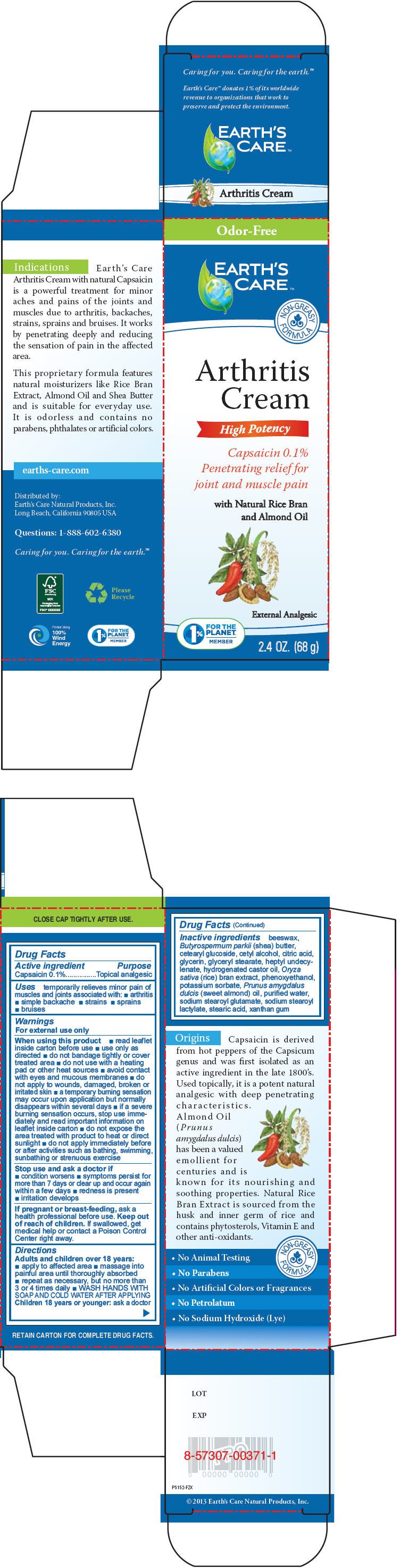
Uses
temporarily relieves minor pain of muscles and joints associated with:
- arthritis
- simple backache
- strains
- sprains
- bruises
Warnings
For external use only
When using this product
- read leaflet inside carton before use
- use only as directed
- do not bandage tightly or cover treated area
- do not use with a heating pad or other heat sources
- avoid contact with eyes and mucous membranes
- do not apply to wounds, damaged, broken or irritated skin
- a temporary burning sensation may occur upon application but normally disappears within several days
- if a severe burning sensation occurs, stop use immediately and read important information on leaflet inside carton
- do not expose the area treated with product to heat or direct sunlight
- do not apply immediately before or after activities such as bathing, swimming, sunbathing or strenuous exercise
Directions
Adults and children over 18 years:
- apply to affected area
- massage into painful area until thoroughly absorbed
- repeat as necessary, but no more than 3 or 4 times daily
- WASH HANDS WITH SOAP AND COLD WATER AFTER APPLYING
Children 18 years or younger: ask a doctor
Inactive ingredients
beeswax, Butyrospermum parkii (shea) butter, cetearyl glucoside, cetyl alcohol, citric acid, glycerin, glyceryl stearate, heptyl undecylenate, hydrogenated castor oil, Oryza sativa (rice) bran extract, phenoxyethanol, potassium sorbate, Prunus amygdalus dulcis (sweet almond) oil, purified water, sodium stearoyl glutamate, sodium stearoyl lactylate, stearic acid, xanthan gum