Label: ROBITUSSIN CHEST CONGESTION- guaifenesin liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 0031-8748-04 - Packager: Wyeth Consumer Healthcare LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated December 4, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
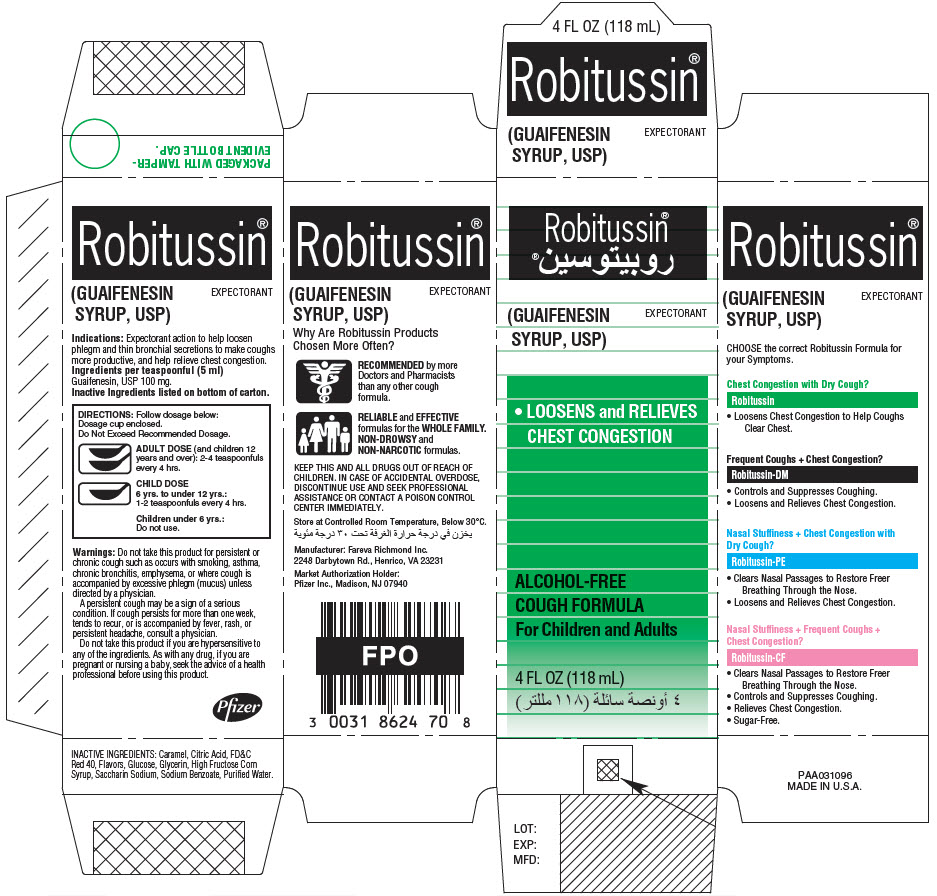
PRINCIPAL DISPLAY PANEL - 118 mL Bottle Label
4 FL OZ (118mL)
Robitussin®
(GUAIFENESIN SYRUP, USP)
EXPECTORANT
DIRECTIONS: Follow dosage below:
Do Not Exceed Recommended Dosage.ADULT DOSE (and children 12 years and
over): 2 - 4 teaspoonfuls every 4 hours.CHILD DOSE
6 yrs. to under 12 yrs.:
1 - 2 teaspoonfuls every 4 hours.Children under 6 yrs.:
Do not use.TAMPER-EVIDENT BOTTLE CAP.
IF BREAKABLE RING IS
SEPARATED, DO NOT USE.
- PRINCIPAL DISPLAY PANEL - 118 mL Bottle Carton
- PRINCIPAL DISPLAY PANEL - Leaflet
-
INGREDIENTS AND APPEARANCE
ROBITUSSIN CHEST CONGESTION
guaifenesin liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0031-8748 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 100 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CARAMEL (UNII: T9D99G2B1R) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM BENZOATE (UNII: OJ245FE5EU) WATER (UNII: 059QF0KO0R) Product Characteristics Color RED Score Shape Size Flavor RASPBERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0031-8748-04 1 in 1 CARTON 09/09/2013 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date EXPORT ONLY 09/21/2004 Labeler - Wyeth Consumer Healthcare LLC (828831730) Registrant - Pfizer Inc (113480771)