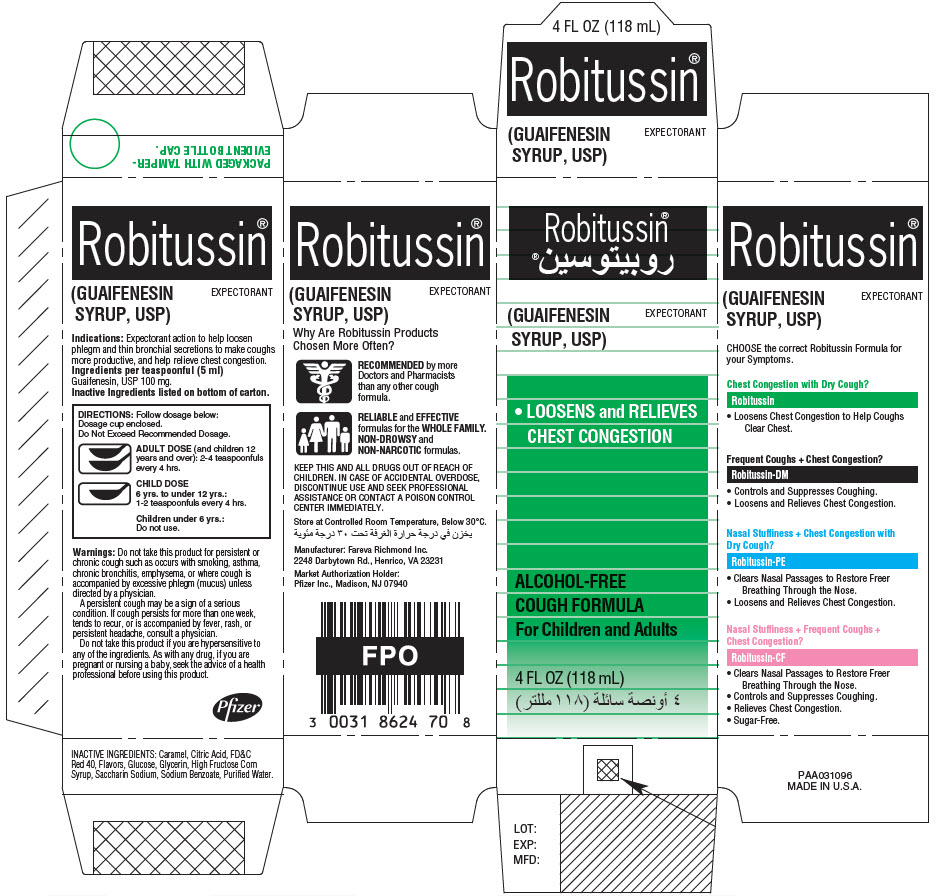
PRINCIPAL DISPLAY PANEL - 118 mL Bottle Label
4 FL OZ (118mL)
Robitussin®
(GUAIFENESIN SYRUP, USP)
EXPECTORANT
DIRECTIONS: Follow dosage below:
Do Not Exceed Recommended Dosage.
ADULT DOSE (and children 12 years and
over): 2 - 4 teaspoonfuls every 4 hours.
CHILD DOSE
6 yrs. to under 12 yrs.:
1 - 2 teaspoonfuls every 4 hours.
Children under 6 yrs.:
Do not use.
TAMPER-EVIDENT BOTTLE CAP.
IF BREAKABLE RING IS
SEPARATED, DO NOT USE.