Label: TRIDERMA PAIN RELIEF- lidocaine hcl, menthol cream
- NDC Code(s): 10738-073-20, 10738-073-21, 10738-073-41
- Packager: Genuine Virgin Aloe Corporation
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 2, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
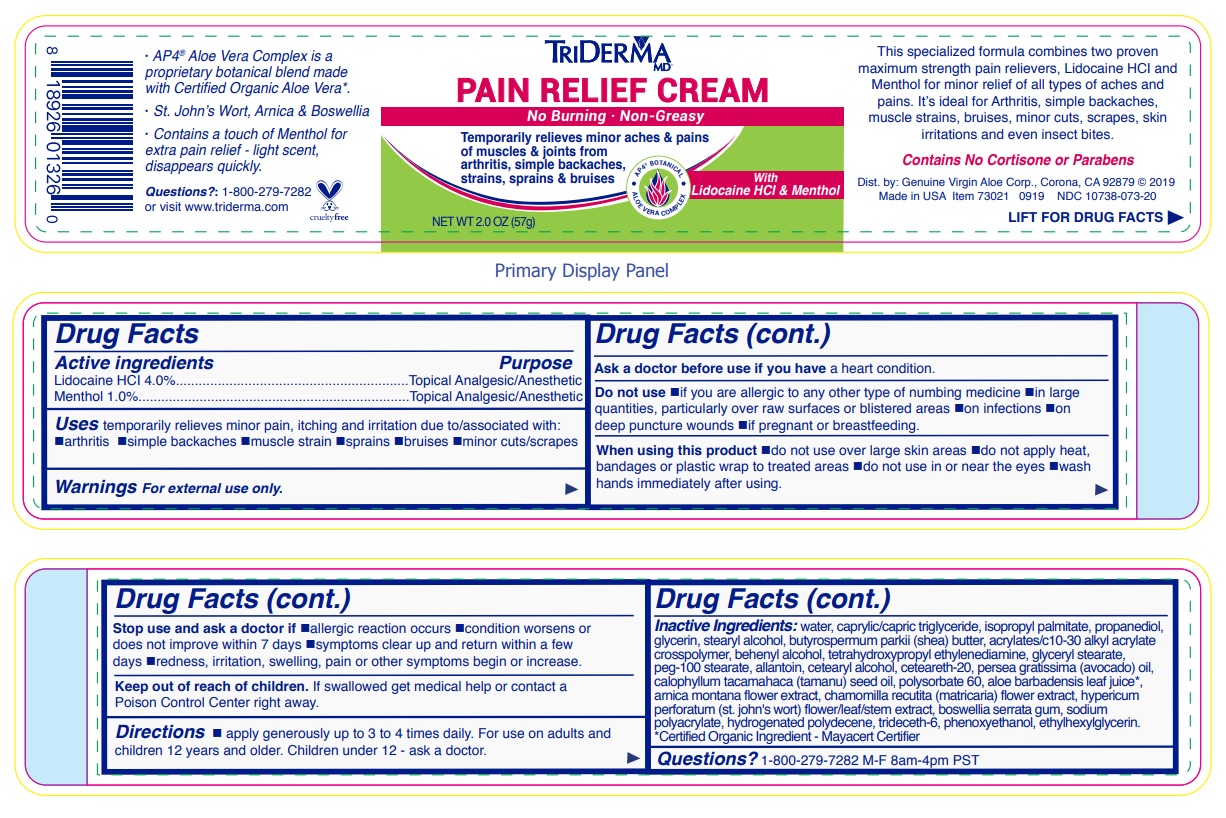
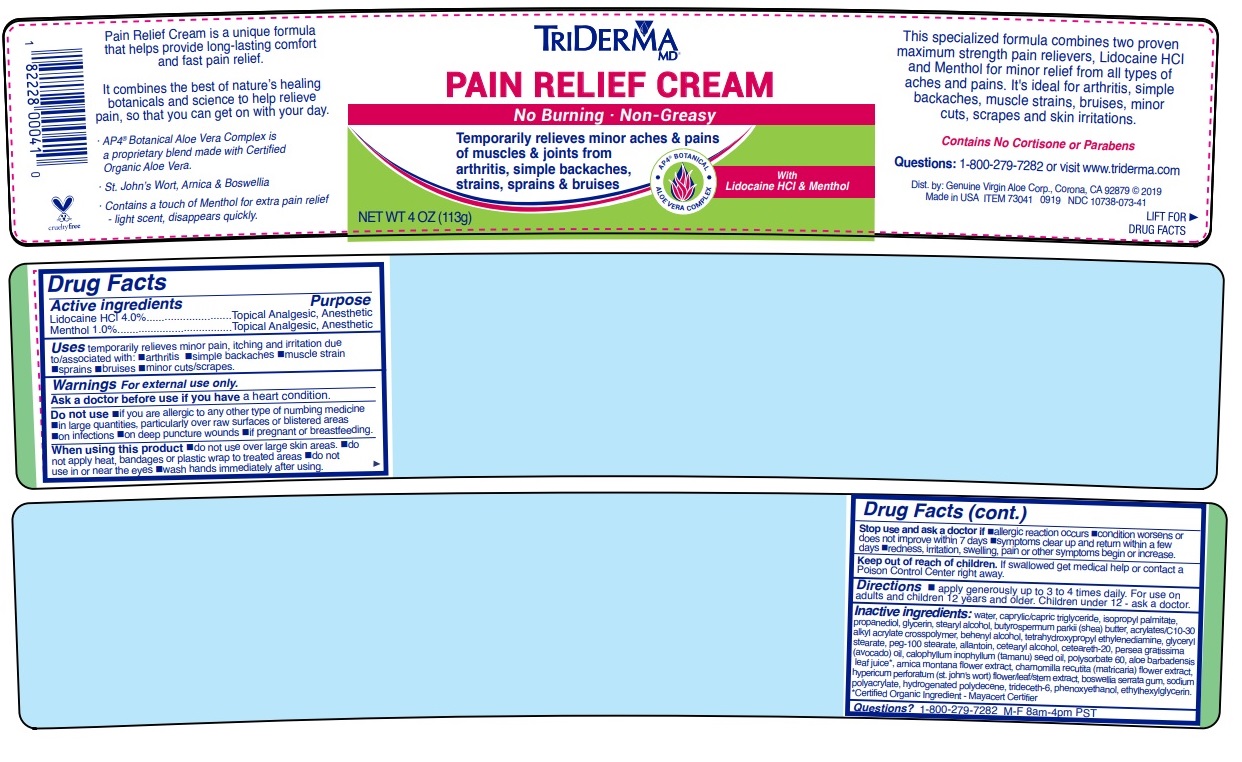
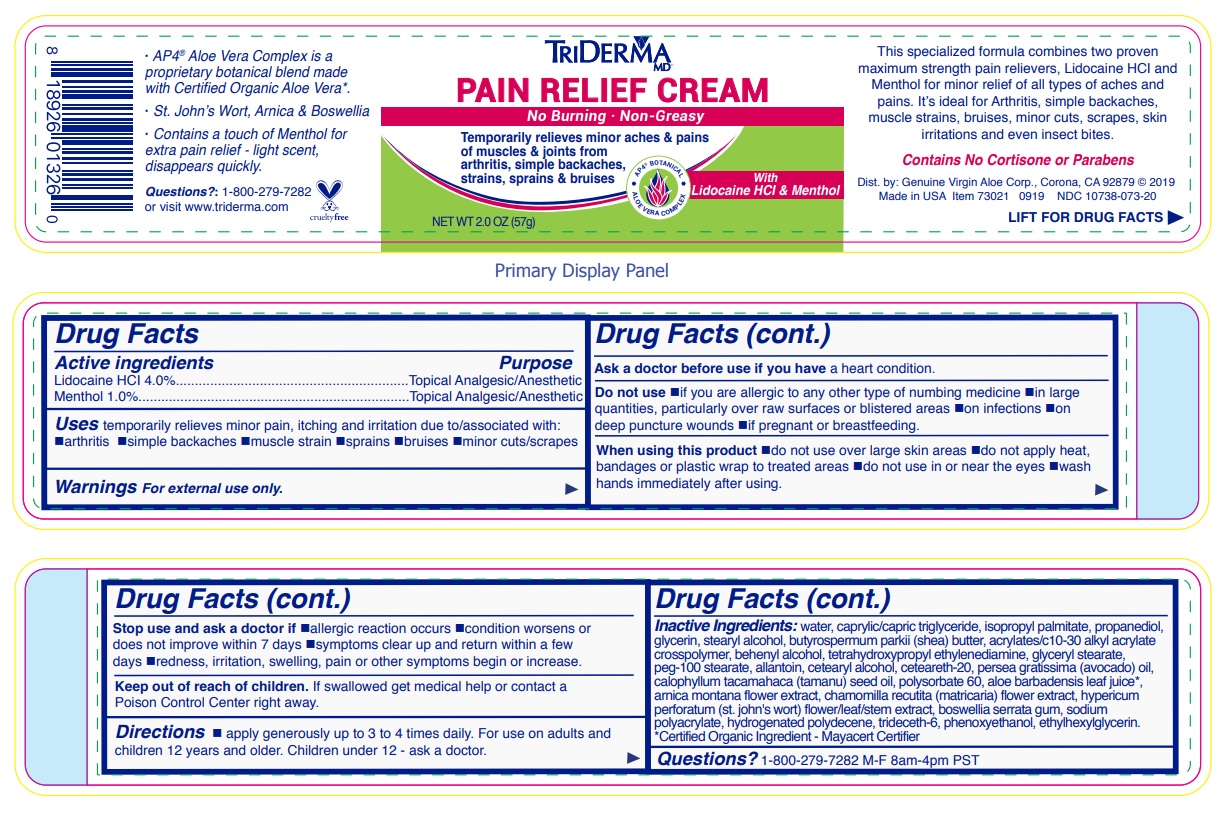
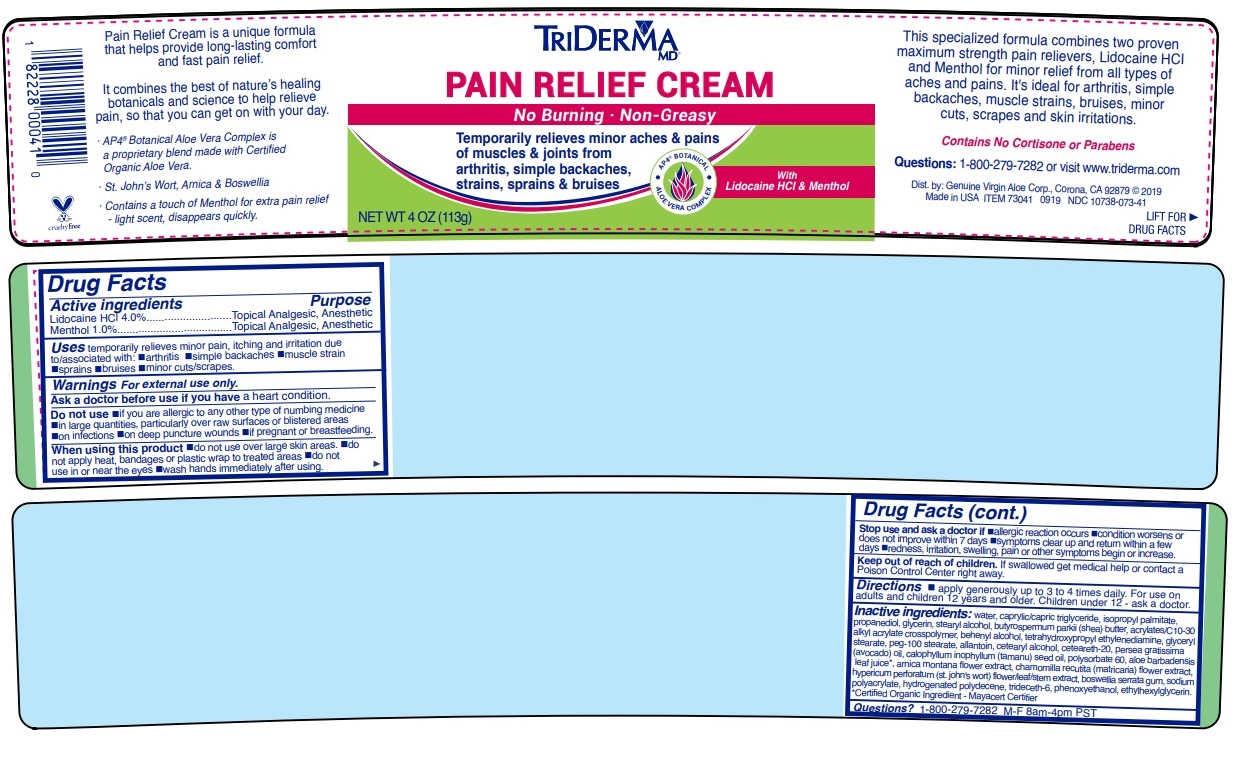
- Drug Facts
- Active ingredients
- Purpose
- INDICATIONS & USAGE
-
WARNINGS
Warnings For external use only.
Ask a doctor before use if you have a heart condition.
Do not use •if you are allergic to any other type of numbing medicine •in large quantities, particularly over raw surfaces or blistered areas •on infections •on deep puncture wounds •if pregnant or breastfeeding.
When using this product •do not use over large skin areas •do not apply heat, bandages or plastic wrap to treated areas •do not use in or near the eyes •wash hands immediately after using.
Stop use and ask a doctor if •allergic reaction occurs •condition worsens or does not improve within 7 days •symptoms clear up and return within a few days •redness, irritation, swelling, pain or other symptoms begin or increase.
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive ingredients: water, caprylic/capric triglyceride, isopropyl palmitate, propanediol, glycerin, stearyl alcohol, butyrospermum parkii (shea) butter, acrylates/c10-30 alkyl acrylate crosspolymer, behenyl alcohol, tetrahydroxypropyl ethylenediamine, glyceryl stearate, peg-100 stearate, allantoin, cetearyl alcohol, ceteareth-20, persea gratissima (avocado) oil, calophyllum inophyllum (tamanu) seed oil, polysorbate 60, aloe barbadensis leaf juice*, arnica montana flower extract, chamomilla recutita (matricaria) flower extract, hypericum perforatum (st. john's wort) flower/leaf/stem extract, boswellia serrata gum, sodium polyacrylate, hydrogenated polydecene, trideceth-6, phenoxyethanol, ethylhexylglycerin.*Certified Organic Ingredient - Mayacert Certifier
- QUESTIONS
-
SPL UNCLASSIFIED SECTION
No Burning · Non-Greasy
Temporarily relieves minor aches & pains of muscles & joints from arthritis, simple backaches, strains, sprains & bruises
AP4 BOTANICAL
ALOE VERA COMPLEX
With Lidocaine HCl & Menthol
This specialized formula combines two proven maximum strength pain relievers, Lidocaine HCl and Menthol for minor relief of all types of aches and pains. It’s ideal for Arthritis, simple backaches, muscle strains, bruises, minor cuts, scrapes, skin irritations and even insect bites.
Contains No Cortisone or Parabens
visit www.triderma.com
Dist. by: Genuine Virgin Aloe Corp., Corona, CA 92879
Made in USAPain Relief Cream is a unique formula that helps provide long-lasting comfort and fast pain relief.
It combines the best of nature’s healing botanicals and science to help relieve pain, so that you can get on with your day.
· AP4 ® Aloe Vera Complex is a proprietary botanical blend made with Certified Organic Aloe Vera*.
· St. John’s Wort, Arnica & Boswellia
· Contains a touch of Menthol for extra pain relief - light scent, disappears quickly.
- Packaging
- Packaging
-
INGREDIENTS AND APPEARANCE
TRIDERMA PAIN RELIEF
lidocaine hcl, menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10738-073 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 4 g in 100 g MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 1 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) PROPANEDIOL (UNII: 5965N8W85T) GLYCERIN (UNII: PDC6A3C0OX) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) SHEA BUTTER (UNII: K49155WL9Y) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) DOCOSANOL (UNII: 9G1OE216XY) EDETOL (UNII: Q4R969U9FR) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) ALLANTOIN (UNII: 344S277G0Z) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) AVOCADO OIL (UNII: 6VNO72PFC1) TAMANU OIL (UNII: JT3LVK84A1) POLYSORBATE 60 (UNII: CAL22UVI4M) ALOE VERA LEAF (UNII: ZY81Z83H0X) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) CHAMOMILE (UNII: FGL3685T2X) ST. JOHN'S WORT (UNII: UFH8805FKA) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) HYDROGENATED POLYDECENE (550 MW) (UNII: U333RI6EB7) TRIDECETH-6 (UNII: 3T5PCR2H0C) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10738-073-20 1 in 1 CARTON 08/30/2019 1 NDC:10738-073-21 57 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:10738-073-41 113 g in 1 JAR; Type 0: Not a Combination Product 08/30/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 08/30/2019 Labeler - Genuine Virgin Aloe Corporation (961374147) Establishment Name Address ID/FEI Business Operations Genuine Virgin Aloe Corporation 961374147 manufacture(10738-073)