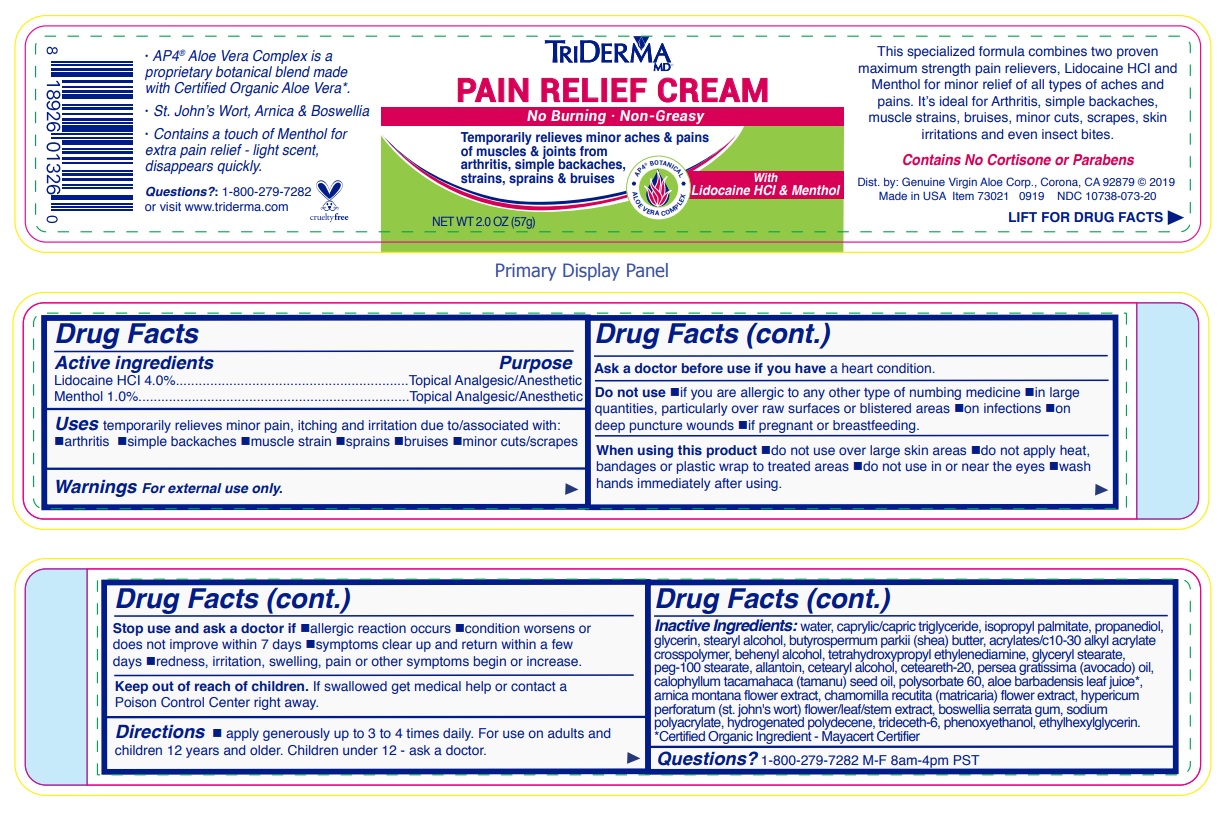
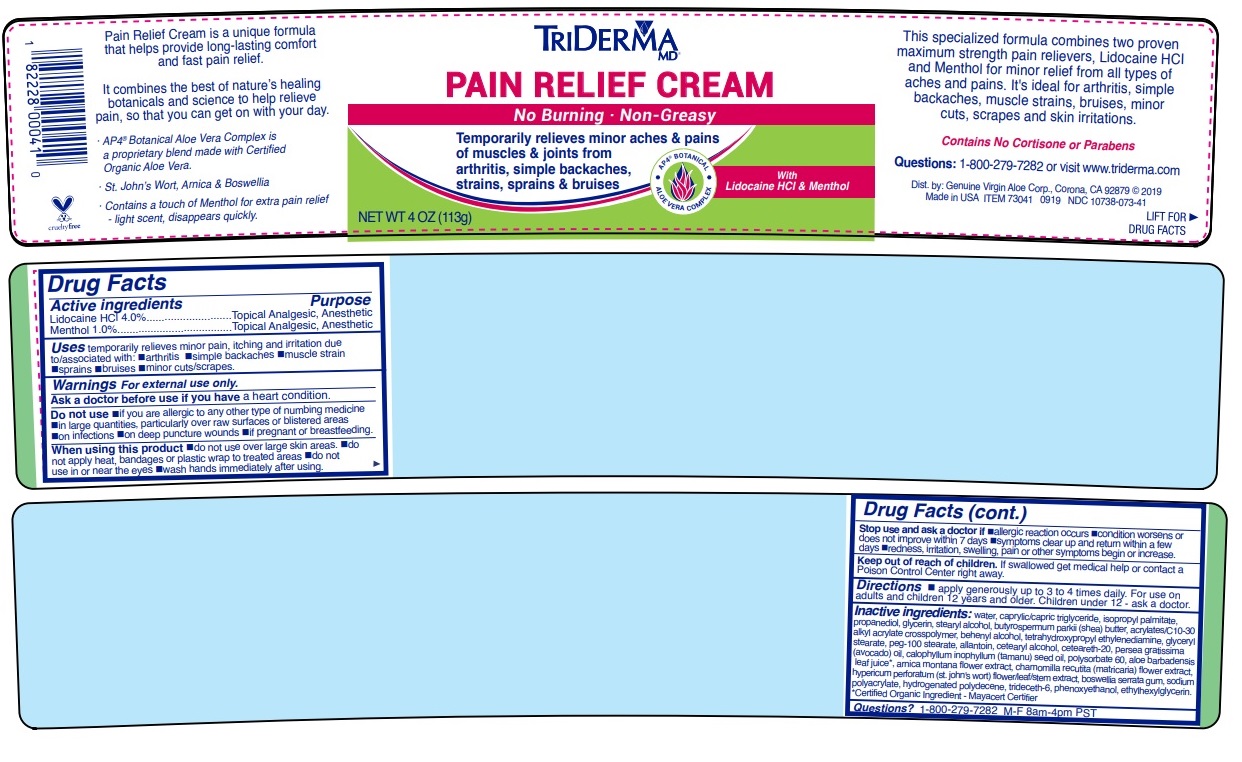
Uses temporarily relieves minor pain, itching and irritation due to/associated with: •arthritis •simple backaches •muscle strain •sprains •bruises •minor cuts/scrapes
Warnings For external use only.
Ask a doctor before use if you have a heart condition.
Do not use •if you are allergic to any other type of numbing medicine •in large quantities, particularly over raw surfaces or blistered areas •on infections •on deep puncture wounds •if pregnant or breastfeeding.
When using this product •do not use over large skin areas •do not apply heat, bandages or plastic wrap to treated areas •do not use in or near the eyes •wash hands immediately after using.
Stop use and ask a doctor if •allergic reaction occurs •condition worsens or does not improve within 7 days •symptoms clear up and return within a few days •redness, irritation, swelling, pain or other symptoms begin or increase.
Directions • apply generously up to 3 to 4 times daily. For use on adults and children 12 years and older. Children under 12 - ask a doctor.
Inactive ingredients: water, caprylic/capric triglyceride, isopropyl palmitate, propanediol, glycerin, stearyl alcohol, butyrospermum parkii (shea) butter, acrylates/c10-30 alkyl acrylate crosspolymer, behenyl alcohol, tetrahydroxypropyl ethylenediamine, glyceryl stearate, peg-100 stearate, allantoin, cetearyl alcohol, ceteareth-20, persea gratissima (avocado) oil, calophyllum inophyllum (tamanu) seed oil, polysorbate 60, aloe barbadensis leaf juice*, arnica montana flower extract, chamomilla recutita (matricaria) flower extract, hypericum perforatum (st. john's wort) flower/leaf/stem extract, boswellia serrata gum, sodium polyacrylate, hydrogenated polydecene, trideceth-6, phenoxyethanol, ethylhexylglycerin.*Certified Organic Ingredient - Mayacert Certifier
No Burning · Non-Greasy
Temporarily relieves minor aches & pains of muscles & joints from arthritis, simple backaches, strains, sprains & bruises
AP4 BOTANICAL
ALOE VERA COMPLEX
With Lidocaine HCl & Menthol
This specialized formula combines two proven maximum strength pain relievers, Lidocaine HCl and Menthol for minor relief of all types of aches and pains. It’s ideal for Arthritis, simple backaches, muscle strains, bruises, minor cuts, scrapes, skin irritations and even insect bites.
Contains No Cortisone or Parabens
visit www.triderma.com
Dist. by: Genuine Virgin Aloe Corp., Corona, CA 92879
Made in USA
Pain Relief Cream is a unique formula that helps provide long-lasting comfort and fast pain relief.
It combines the best of nature’s healing botanicals and science to help relieve pain, so that you can get on with your day.
· AP4 ® Aloe Vera Complex is a proprietary botanical blend made with Certified Organic Aloe Vera*.
· St. John’s Wort, Arnica & Boswellia
· Contains a touch of Menthol for extra pain relief - light scent, disappears quickly.