Label: DUAL ACTION COMPLETE- famotidine, calcium carbonate and magnesium hydroxide tablet, chewable
- NDC Code(s): 56062-263-63, 56062-263-71
- Packager: Publix Super Markets Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 12, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
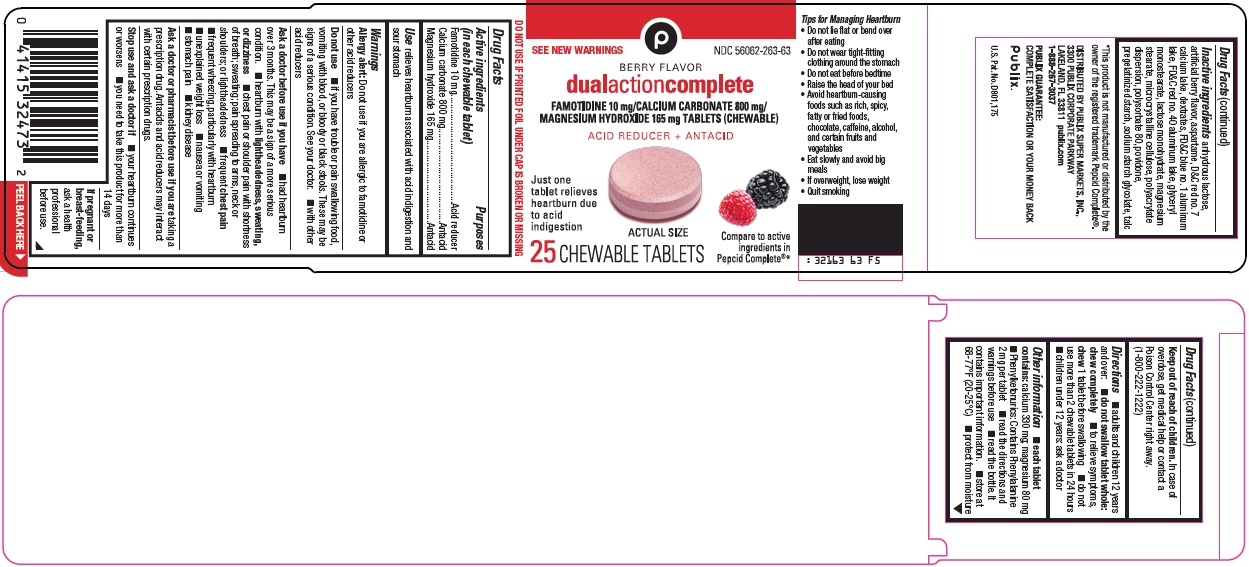
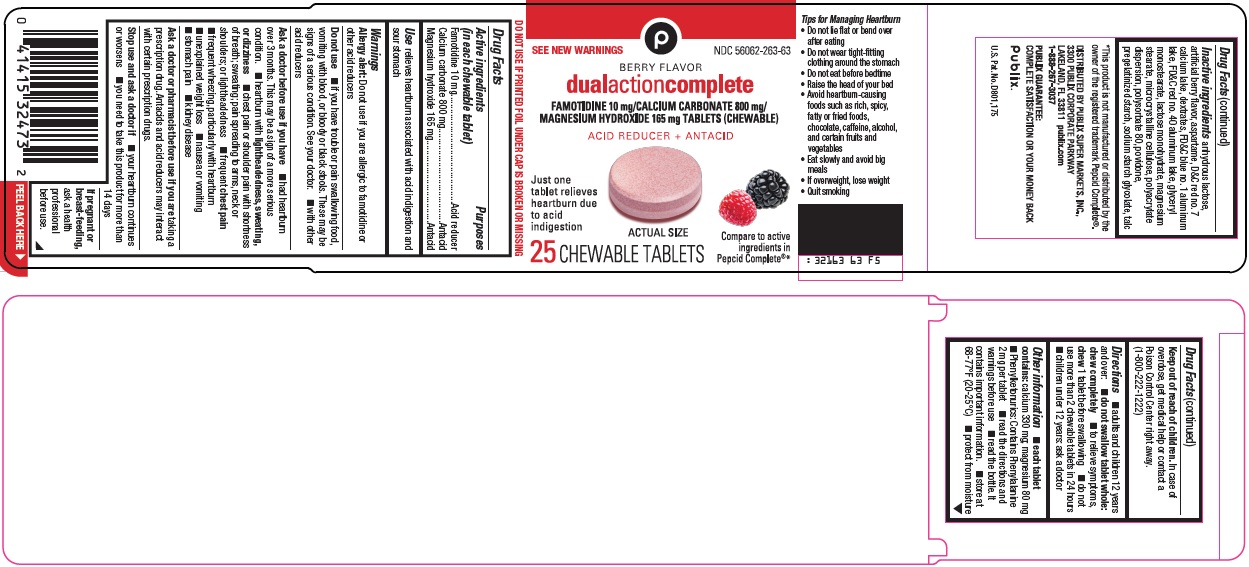
- Active ingredients (in each chewable tablet)
- Purposes
- Use
-
Warnings
Allergy alert: Do not use if you are allergic to famotidine or other acid reducers
Do not use
- •
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
- •
- with other acid reducers
Ask a doctor before use if you have
- •
- had heartburn over 3 months. This may be a sign of a more serious condition.
- •
- heartburn with lightheadedness, sweating, or dizziness
- •
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- •
- frequent chest pain
- •
- frequent wheezing, particularly with heartburn
- •
- unexplained weight loss
- •
- nausea or vomiting
- •
- stomach pain
- •
- kidney disease
Ask a doctor or pharmacist before use if you are
taking a prescription drug. Antacids and acid reducers may interact with certain prescription drugs.
- Directions
- Other information
-
Inactive ingredients
anhydrous lactose, artificial berry flavor, aspartame, D&C red no. 7 calcium lake, dextrates, FD&C blue no. 1 aluminum lake, FD&C red no. 40 aluminum lake, glyceryl monostearate, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyacrylate dispersion, polysorbate 80, povidone, pregelatinized starch, sodium starch glycolate, talc
-
Package/Label Principal Display Panel
SEE NEW WARNINGS
BERRY FLAVOR
dual action complete
FAMOTIDINE 10 mg/CALCIUM CARBONATE 800 mg/MAGNESIUM HYDROXIDE 165 mg TABLETS (CHEWABLE)
ACID REDUCER + ANTACID
Just one tablet relieves heartburn due to acid indigestion
ACTUAL SIZE
Compare to active ingredients in Pepcid Complete®
25 CHEWABLE TABLETS
-
INGREDIENTS AND APPEARANCE
DUAL ACTION COMPLETE
famotidine, calcium carbonate and magnesium hydroxide tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:56062-263 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FAMOTIDINE (UNII: 5QZO15J2Z8) (FAMOTIDINE - UNII:5QZO15J2Z8) FAMOTIDINE 10 mg CALCIUM CARBONATE (UNII: H0G9379FGK) (CARBONATE ION - UNII:7UJQ5OPE7D, CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CARBONATE 800 mg MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) (MAGNESIUM CATION - UNII:T6V3LHY838, HYDROXIDE ION - UNII:9159UV381P) MAGNESIUM HYDROXIDE 165 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) ASPARTAME (UNII: Z0H242BBR1) D&C RED NO. 7 (UNII: ECW0LZ41X8) DEXTRATES (UNII: G263MI44RU) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color PINK (mottled) Score no score Shape ROUND (bi-layered) Size 17mm Flavor BERRY Imprint Code L321 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:56062-263-63 25 in 1 BOTTLE; Type 0: Not a Combination Product 11/06/2018 2 NDC:56062-263-71 50 in 1 BOTTLE; Type 0: Not a Combination Product 11/06/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077355 11/06/2018 Labeler - Publix Super Markets Inc (006922009)