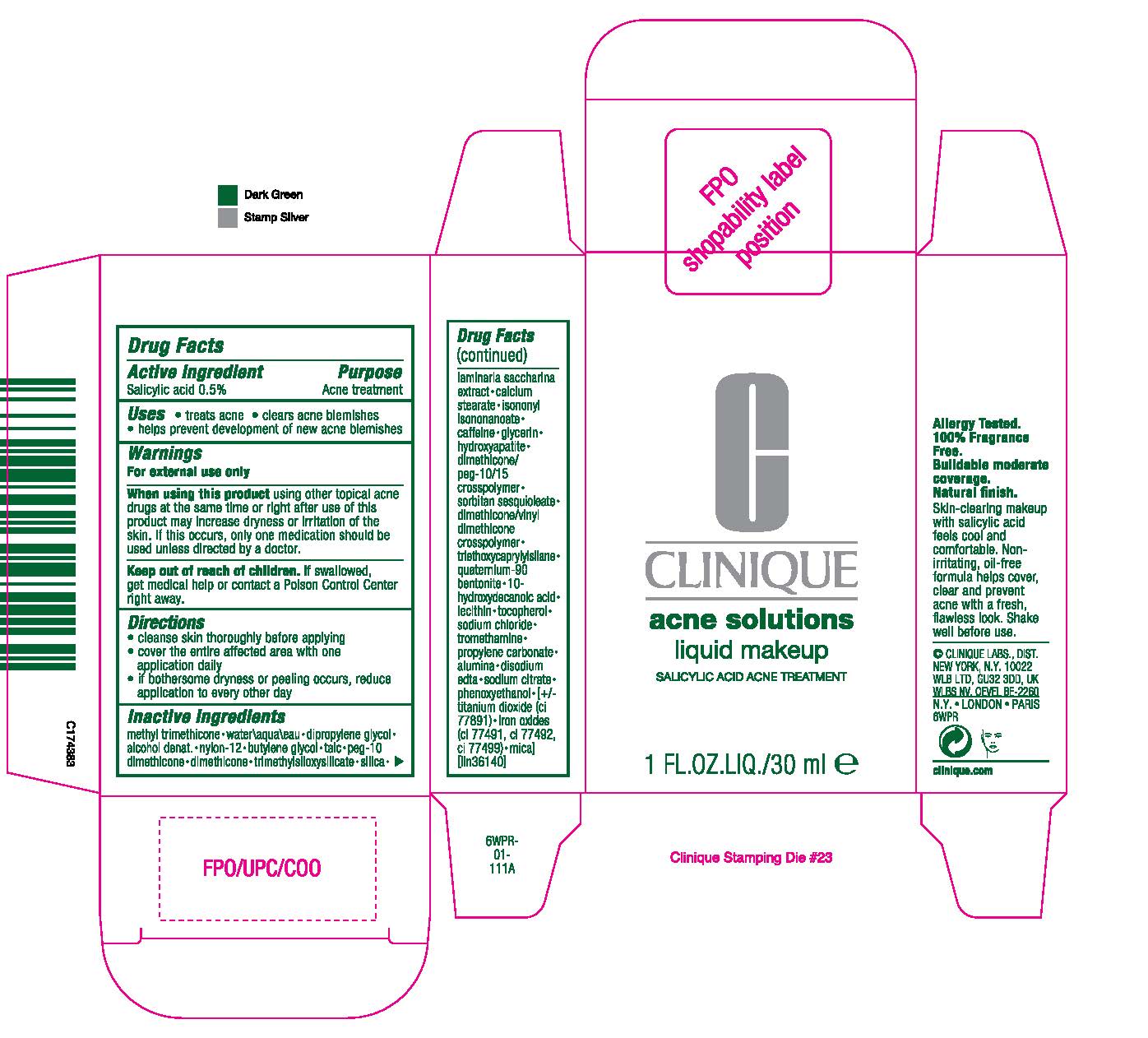
Label: ACNE SOLUTIONS LIQUID MAKEUP SALICYLIC ACID ACNE TREATMENT- salicylic acid liquid
- NDC Code(s): 49527-751-01
- Packager: CLINIQUE LABORATORIES LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- WHEN USING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
INACTIVE INGREDIENTS:
METHYL TRIMETHICONE [] WATER [] DIPROPYLENE GLYCOL [] ALCOHOL DENAT. [] NYLON-12 [] BUTYLENE GLYCOL [] TALC [] PEG-10 DIMETHICONE [] DIMETHICONE [] TRIMETHYLSILOXYSILICATE [] SILICA [] LAMINARIA SACCHARINA EXTRACT [] CALCIUM STEARATE [] ISONONYL ISONONANOATE [] CAFFEINE [] GLYCERIN [] HYDROXYAPATITE [] DIMETHICONE/PEG-10/15 CROSSPOLYMER [] SORBITAN SESQUIOLEATE [] DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER [] TRIETHOXYCAPRYLYLSILANE [] QUATERNIUM-90 BENTONITE [] 10-HYDROXYDECANOIC ACID [] LECITHIN [] TOCOPHEROL [] SODIUM CHLORIDE [] TROMETHAMINE [] PROPYLENE CARBONATE [] ALUMINA [] DISODIUM EDTA [] SODIUM CITRATE [] PHENOXYETHANOL MAY CONTAIN: TITANIUM DIOXIDE [] IRON OXIDES[] MICA ILN36140
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACNE SOLUTIONS LIQUID MAKEUP SALICYLIC ACID ACNE TREATMENT
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49527-751 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength PROPYLENE CARBONATE (UNII: 8D08K3S51E) ALUMINUM OXIDE (UNII: LMI26O6933) DISODIUM EDTA-COPPER (UNII: 6V475AX06U) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) PHENOXYETHANOL (UNII: HIE492ZZ3T) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICA (UNII: V8A1AW0880) METHYL TRIMETHICONE (UNII: S73ZQI0GXM) WATER (UNII: 059QF0KO0R) DIPROPYLENE GLYCOL (UNII: E107L85C40) ALCOHOL (UNII: 3K9958V90M) NYLON-12 (UNII: 446U8J075B) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TALC (UNII: 7SEV7J4R1U) BIS-PEG-10 DIMETHICONE/DIMER DILINOLEATE COPOLYMER (UNII: CF5W1YCX11) DIMETHICONE (UNII: 92RU3N3Y1O) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) SACCHARINA LATISSIMA (UNII: 68CMP2MB55) CALCIUM STEARATE (UNII: 776XM7047L) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CAFFEINE (UNII: 3G6A5W338E) GLYCERIN (UNII: PDC6A3C0OX) TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (HARD PARTICLE) (UNII: H895X08VNQ) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BENTONITE (UNII: A3N5ZCN45C) 10-HYDROXYDECANOIC ACID (UNII: NP03XO416B) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TOCOPHEROL (UNII: R0ZB2556P8) SODIUM CHLORIDE (UNII: 451W47IQ8X) TROMETHAMINE (UNII: 023C2WHX2V) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49527-751-01 1 in 1 CARTON 12/04/2020 1 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M006 12/04/2020 Labeler - CLINIQUE LABORATORIES LLC (044475127) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations PALC 078364654 pack(49527-751) , label(49527-751) Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics Ltd. 204132062 pack(49527-751) , label(49527-751) Establishment Name Address ID/FEI Business Operations Whitman Laboratories Ltd. 216866277 manufacture(49527-751) , pack(49527-751) , label(49527-751) Establishment Name Address ID/FEI Business Operations Estee Lauder N.V. 370151326 manufacture(49527-751) , pack(49527-751) , label(49527-751) Establishment Name Address ID/FEI Business Operations The Estee Lauder Inc 802599436 manufacture(49527-751) , pack(49527-751) , label(49527-751) Establishment Name Address ID/FEI Business Operations Northtec LLC 943871157 pack(49527-751) , label(49527-751) Establishment Name Address ID/FEI Business Operations PADC 1 949264774 pack(49527-751) , label(49527-751)