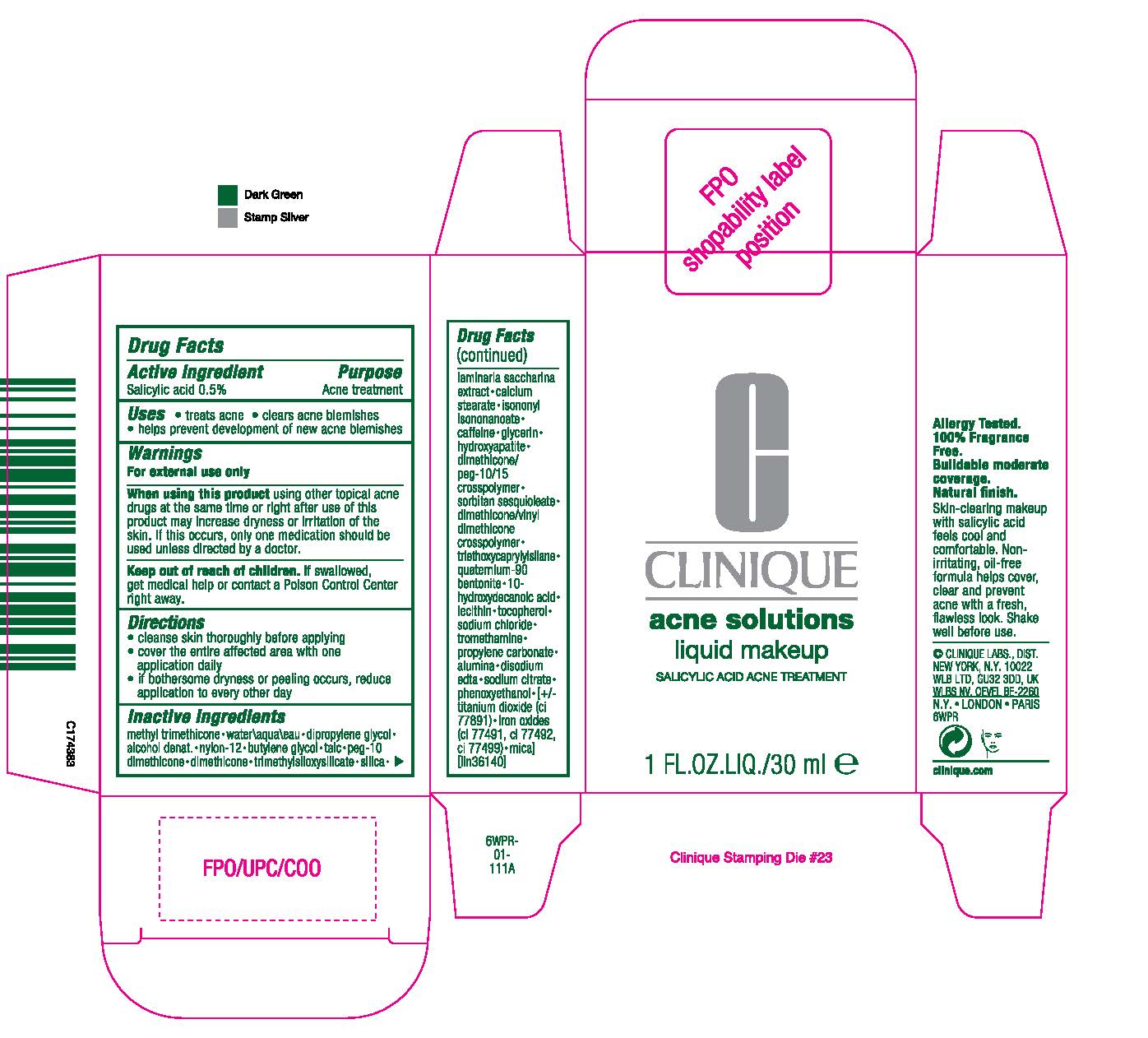
WHEN USING THIS PRODUCT: USING OTHER TOPICAL ACNE DRUGS AT THE SAME TIME OR RIGHT AFTER USE OF THIS PRODUCT MAY INCREASE DRYNESS OR IRRITATION OF THE SKIN. IF THIS OCCURS, ONLY ONE MEDICATION SHOULD BE USED UNLESS DIRECTED BY A DOCTOR.
KEEP OUT OF REACH OF CHILDREN. IF SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
DIRECTIONS
- SHAKE WELL BEFORE USE
- CLEANSE SKIN THOROUGHLY BEFORE APPLYING
- COVER THE ENTIRE AFFECTED AREA WITH ONE APPLICATION
- GRADUALLY INCREASE TO 2 OR 3 TIMES DAILY IF NEEDED OR AS DIRECTED BY A DOCTOR
- IF BOTHERSOME DRYNESS OR PEELING OCCURS, REDUCE APPLICATION TO EVERY OTHER DAY.
INACTIVE INGREDIENTS:
METHYL TRIMETHICONE [] WATER [] DIPROPYLENE GLYCOL [] ALCOHOL DENAT. [] NYLON-12 [] BUTYLENE GLYCOL [] TALC [] PEG-10 DIMETHICONE [] DIMETHICONE [] TRIMETHYLSILOXYSILICATE [] SILICA [] LAMINARIA SACCHARINA EXTRACT [] CALCIUM STEARATE [] ISONONYL ISONONANOATE [] CAFFEINE [] GLYCERIN [] HYDROXYAPATITE [] DIMETHICONE/PEG-10/15 CROSSPOLYMER [] SORBITAN SESQUIOLEATE [] DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER [] TRIETHOXYCAPRYLYLSILANE [] QUATERNIUM-90 BENTONITE [] 10-HYDROXYDECANOIC ACID [] LECITHIN [] TOCOPHEROL [] SODIUM CHLORIDE [] TROMETHAMINE [] PROPYLENE CARBONATE [] ALUMINA [] DISODIUM EDTA [] SODIUM CITRATE [] PHENOXYETHANOL MAY CONTAIN: TITANIUM DIOXIDE [] IRON OXIDES[] MICA ILN36140