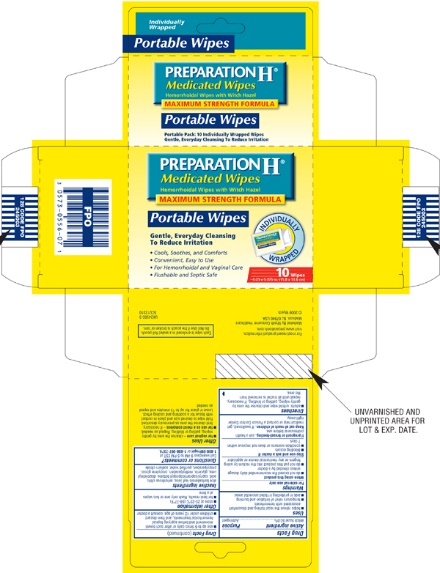
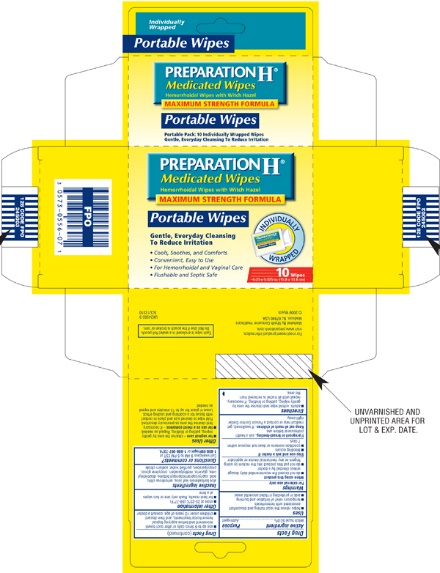
Label: PREPARATION H MEDICATED WIPES- witch hazel cloth
- NDC Code(s): 0573-0556-07, 0573-0556-20, 0573-0556-96
- Packager: Haleon US Holdings LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- USES
- WARNINGS
-
DIRECTIONS
- open the lid on the top of the wipes pouch
- peel back wipes seal, remove completely and discard
- grab the top wipe at the edge of the center fold and pull out of pouch
- close lid after each use to retain moisture
- adults: unfold wipe and cleanse the area by gently wiping, patting or blotting. If necessary, repeat until all matter is removed from the area
- use up to 6 times daily or after each bowel movement and before applying topical hemorrhoidal treatments, and then discard
- children under 12 years of age: consult a doctor
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS OR COMMENTS?
-
PRODUCT PACKAGING
The product packaging shown below represents a sample of that currently in use. Additional packaging may also be available.
PREPARATION H Medicated Wipes
Hemorrhoidal Wipes with Witch Hazel
MAXIMUM STRENGTH FORMULA
Gentle, Everyday Cleansing To Reduce Irritation
48 Medicated Wipes With Aloe
Cools, Soothes and Comforts
Convenient, Easy to Use
For Hemorrhoidal and Vaginal Care
Flushable and Septic Safe
6 x 5 in. (15.2 x 12.7 cm)
For most recent product information, visit www.preparationh.com
Marketed By Wyeth Consumer Healthcare
Madison, NJ 07940 USA
Made in USA ©2009 Wyeth
10 Wipes Individually Wrapped
Each wipe is enclosed in a sealed foil pouch. Do not use if the pouch is broken or torn.
Other Uses
- for vaginal care– cleanse the area by gently wiping, patting or blotting. Repeat as needed.
- for use as a moist compress– if necessary, first cleanse the area as previously described. Fold new wipe to desired size and place in contact with tissue for a soothing and cooling effect. Leave in place for up to 15 minutes and repeat as needed.
-
INGREDIENTS AND APPEARANCE
PREPARATION H MEDICATED WIPES
witch hazel clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0573-0556 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WITCH HAZEL (UNII: 101I4J0U34) (WITCH HAZEL - UNII:101I4J0U34) WITCH HAZEL 5 g Inactive Ingredients Ingredient Name Strength METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CAPRYL/CAPRAMIDOPROPYL BETAINE (UNII: 231H3ZT9NE) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0573-0556-07 10 in 1 CARTON 02/02/2004 1 1 in 1 PACKET; Type 0: Not a Combination Product 2 NDC:0573-0556-96 2 in 1 CARTON 02/02/2004 2 NDC:0573-0556-20 48 in 1 POUCH; Type 0: Not a Combination Product 3 NDC:0573-0556-20 48 in 1 POUCH; Type 0: Not a Combination Product 02/02/2004 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 02/02/2004 Labeler - Haleon US Holdings LLC (079944263)