USES
- helps relieve the local itching and discomfort associated with hemorrhoids
- temporary relief of irritation and burning
- aids in protecting irritated anorectal areas
WARNINGS
For external use only
DIRECTIONS
- open the lid on the top of the wipes pouch
- peel back wipes seal, remove completely and discard
- grab the top wipe at the edge of the center fold and pull out of pouch
- close lid after each use to retain moisture
- adults: unfold wipe and cleanse the area by gently wiping, patting or blotting. If necessary, repeat until all matter is removed from the area
- use up to 6 times daily or after each bowel movement and before applying topical hemorrhoidal treatments, and then discard
- children under 12 years of age: consult a doctor
OTHER INFORMATION
- for best results, flush only one or two wipes at a time
- store at 20-25°C (68-77°F)
INACTIVE INGREDIENTS
aloe barbadensis leaf juice, anhydrous citric acid, capryl/capramidopropyl betaine, diazolidinyl urea, glycerin, methylparaben, propylene glycol, propylparaben, purified water, sodium citrate
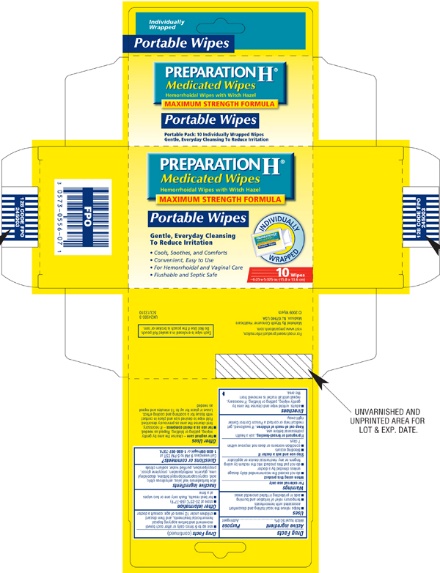
PRODUCT PACKAGING
The product packaging shown below represents a sample of that currently in use. Additional packaging may also be available.
PREPARATION H Medicated Wipes
Hemorrhoidal Wipes with Witch Hazel
MAXIMUM STRENGTH FORMULA
Gentle, Everyday Cleansing To Reduce Irritation
48 Medicated Wipes With Aloe
Cools, Soothes and Comforts
Convenient, Easy to Use
For Hemorrhoidal and Vaginal Care
Flushable and Septic Safe
6 x 5 in. (15.2 x 12.7 cm)
For most recent product information, visit www.preparationh.com
Marketed By Wyeth Consumer Healthcare
Madison, NJ 07940 USA
Made in USA ©2009 Wyeth
10 Wipes Individually Wrapped
Each wipe is enclosed in a sealed foil pouch. Do not use if the pouch is broken or torn.
Other Uses
- for vaginal care– cleanse the area by gently wiping, patting or blotting. Repeat as needed.
- for use as a moist compress– if necessary, first cleanse the area as previously described. Fold new wipe to desired size and place in contact with tissue for a soothing and cooling effect. Leave in place for up to 15 minutes and repeat as needed.