Label: SULWHASOO PERFECTING CUSHION NO. 21- octinoxate, titanium dioxide, and zinc oxide lotion
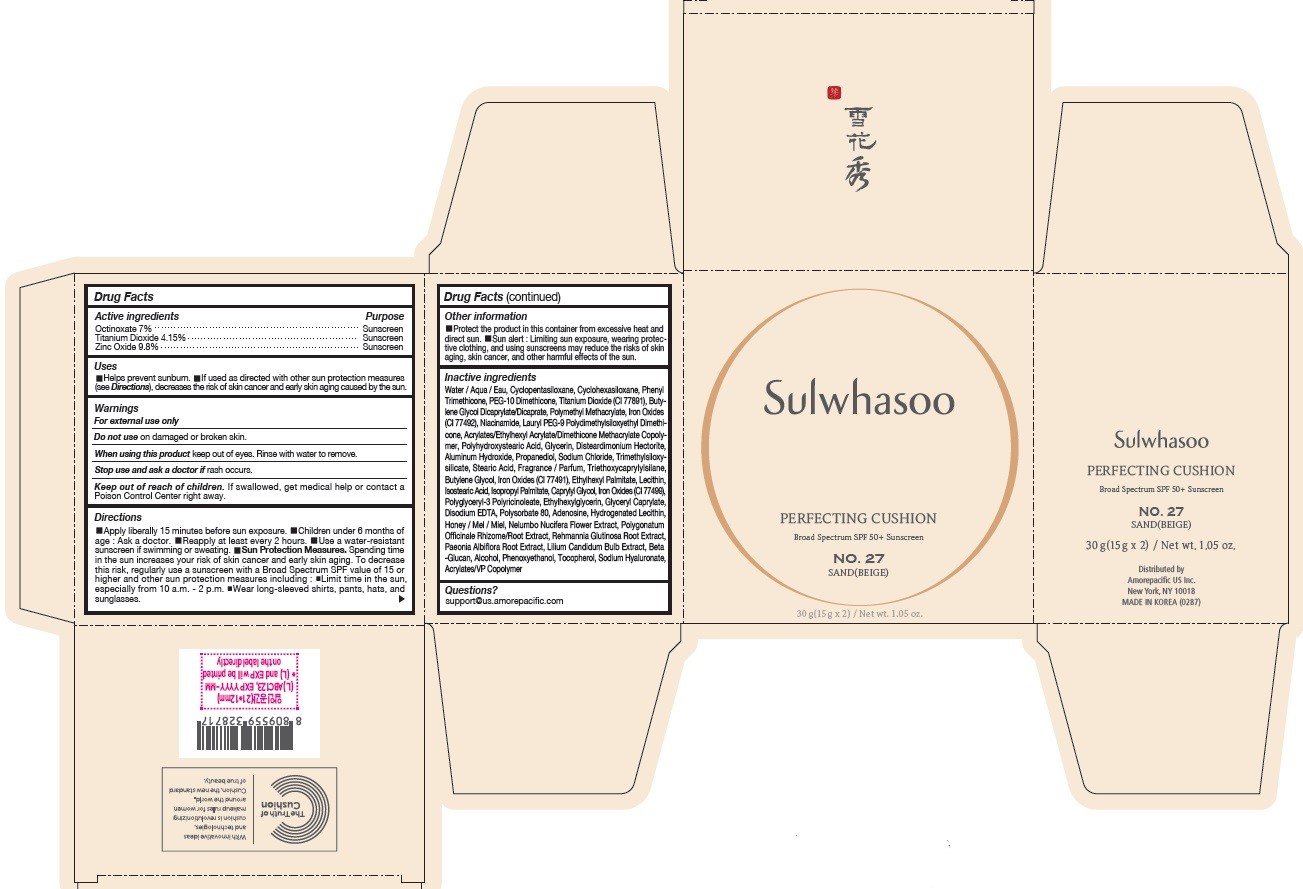
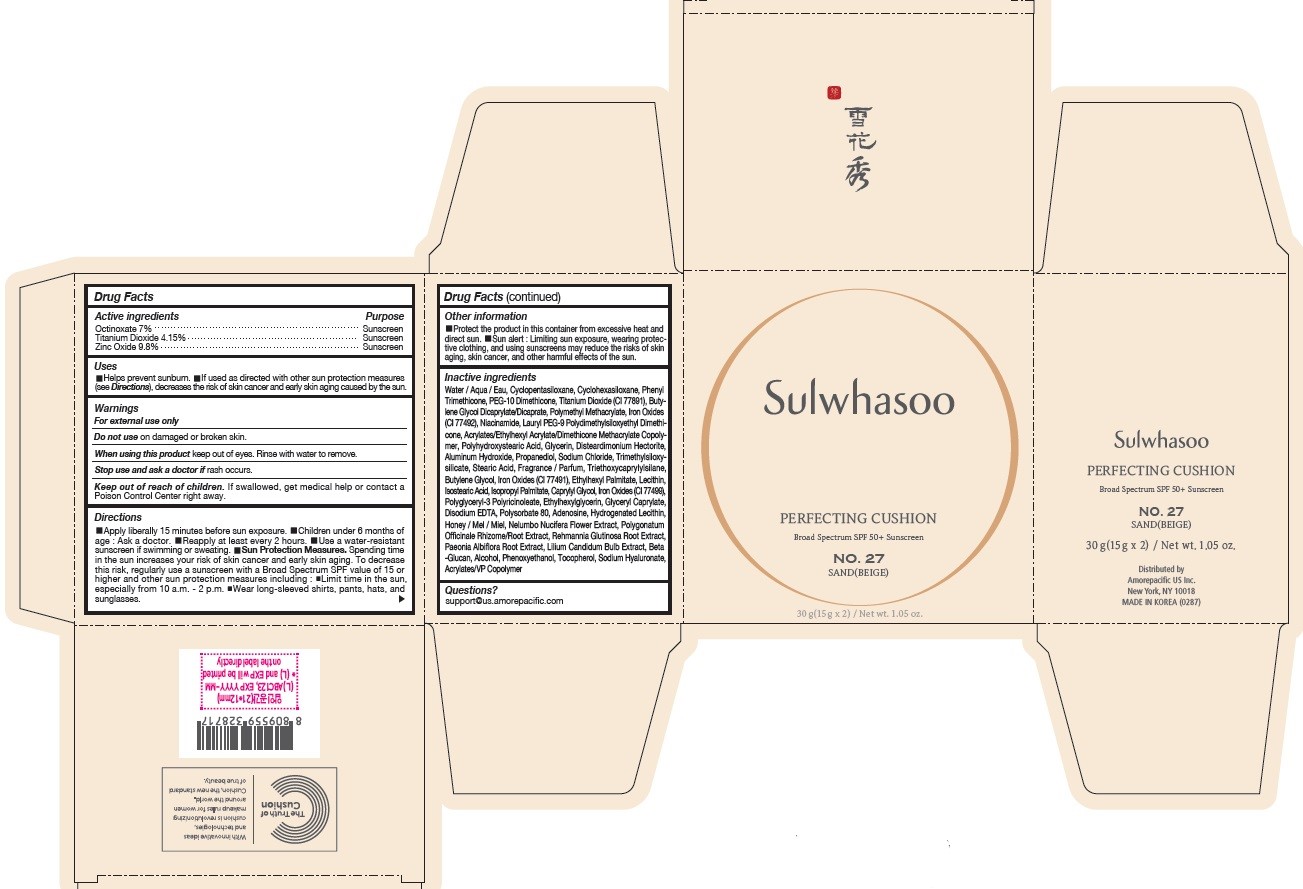
SULWHASOO PERFECTING CUSHION NO. 27- octinoxate, titanium dioxide, and zinc oxide lotion
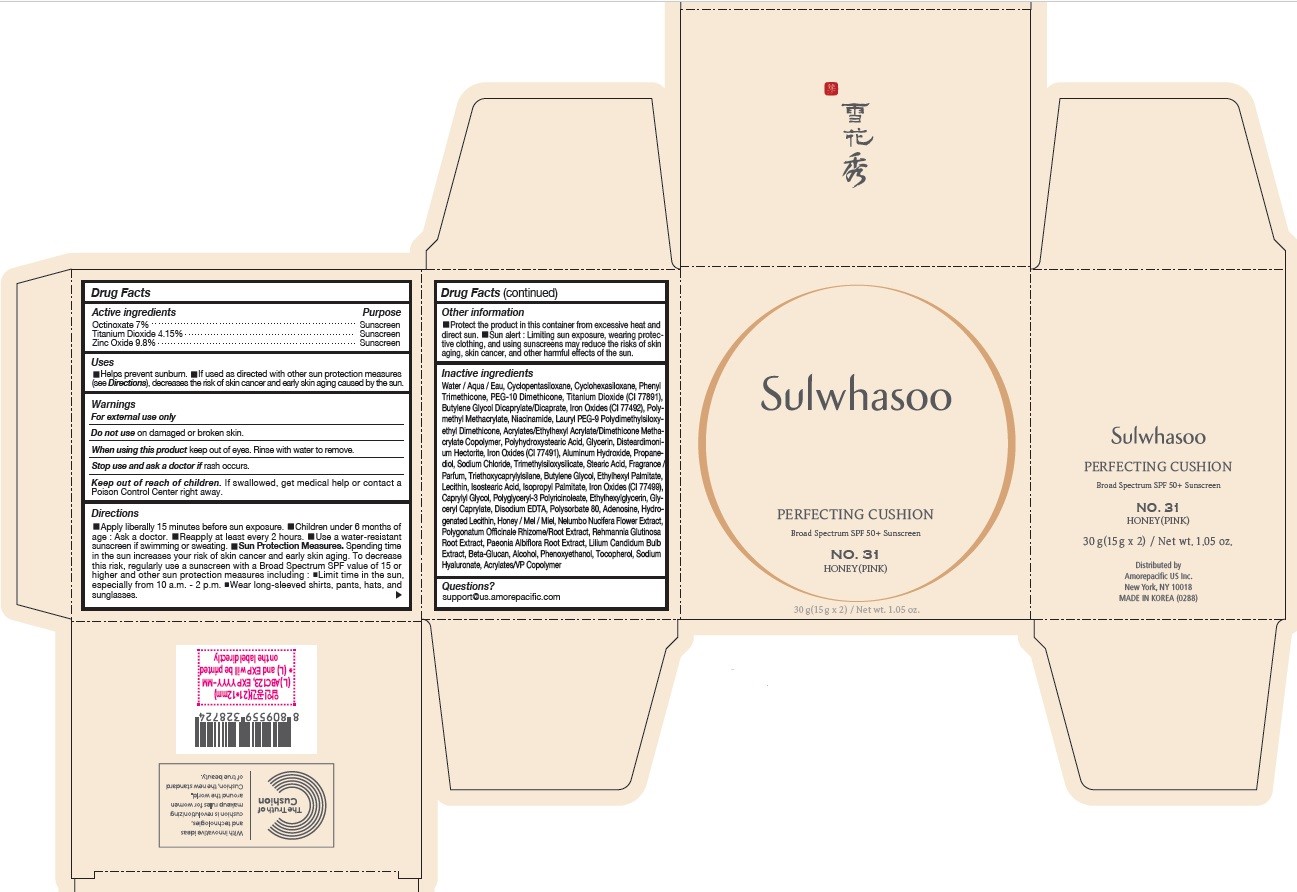
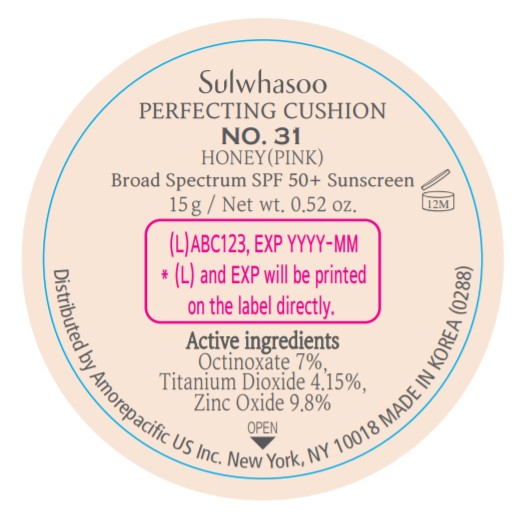
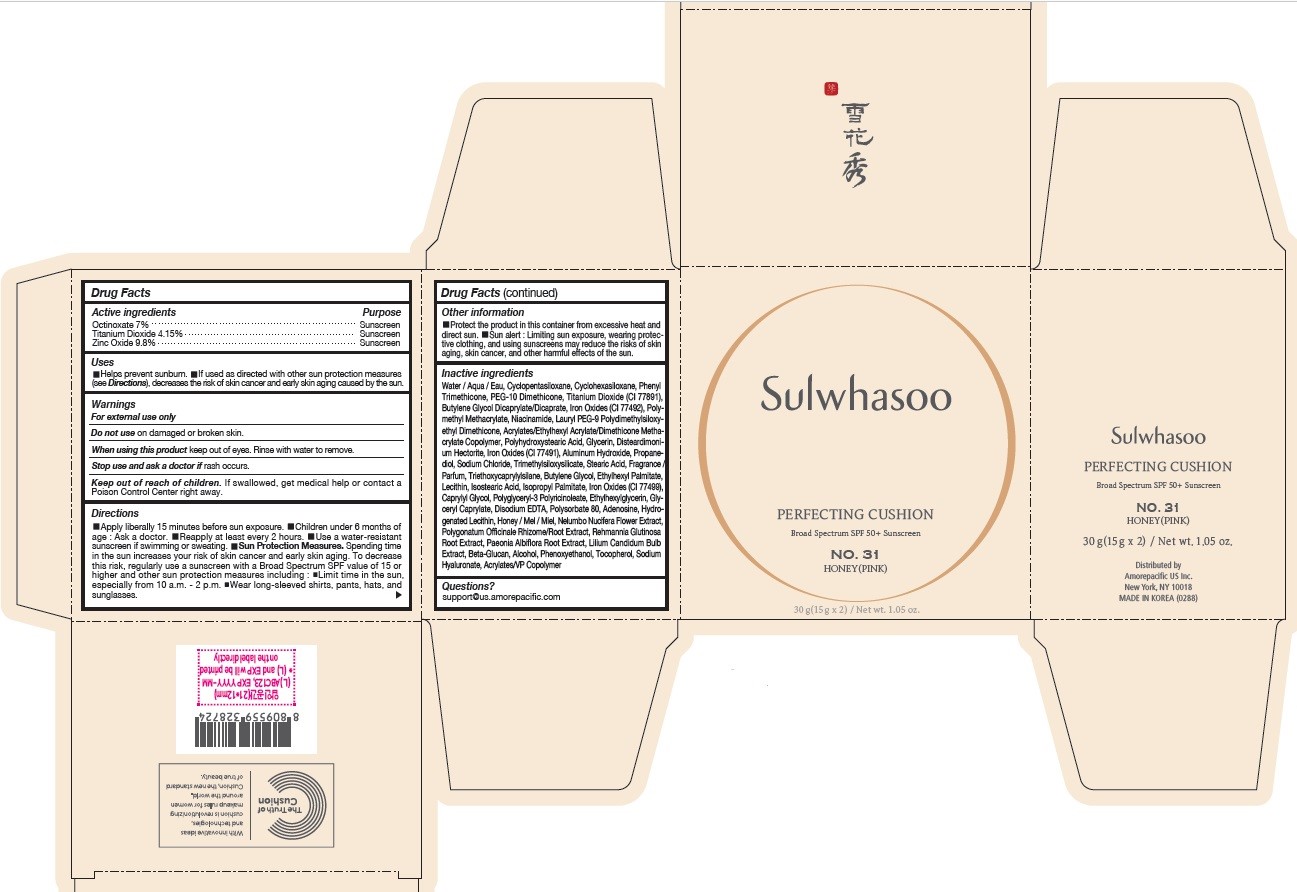
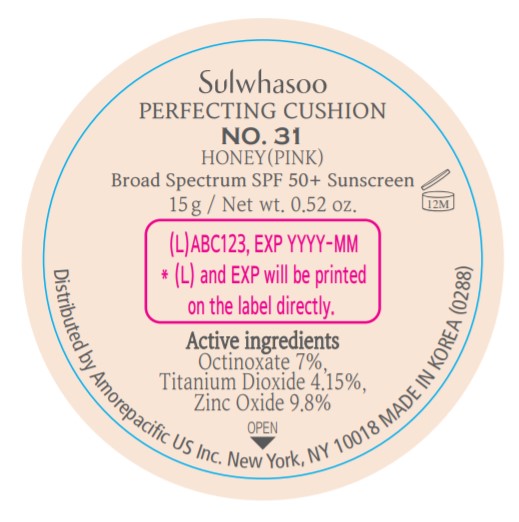
SULWHASOO PERFECTING CUSHION NO. 31- octinoxate, titanium dioxide, and zinc oxide lotion
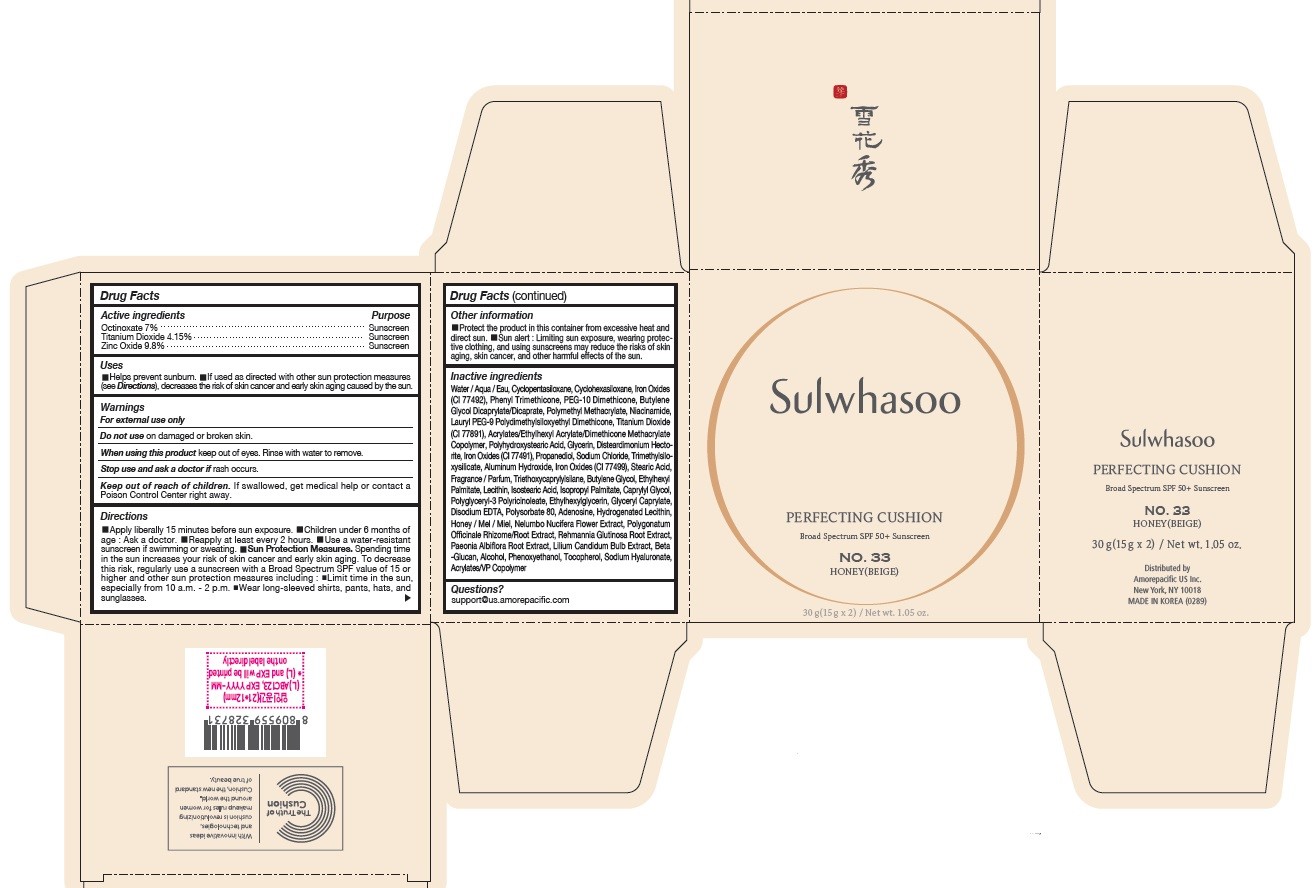
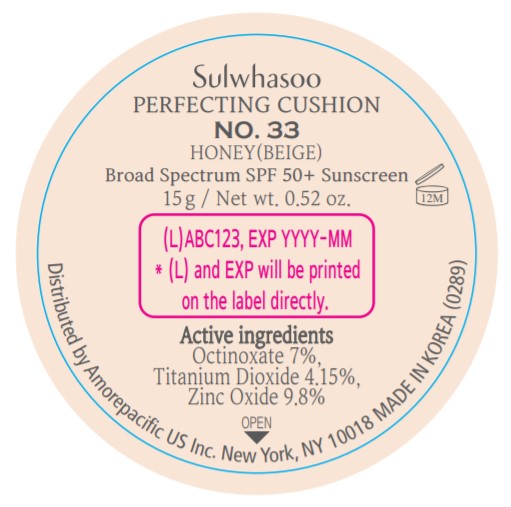
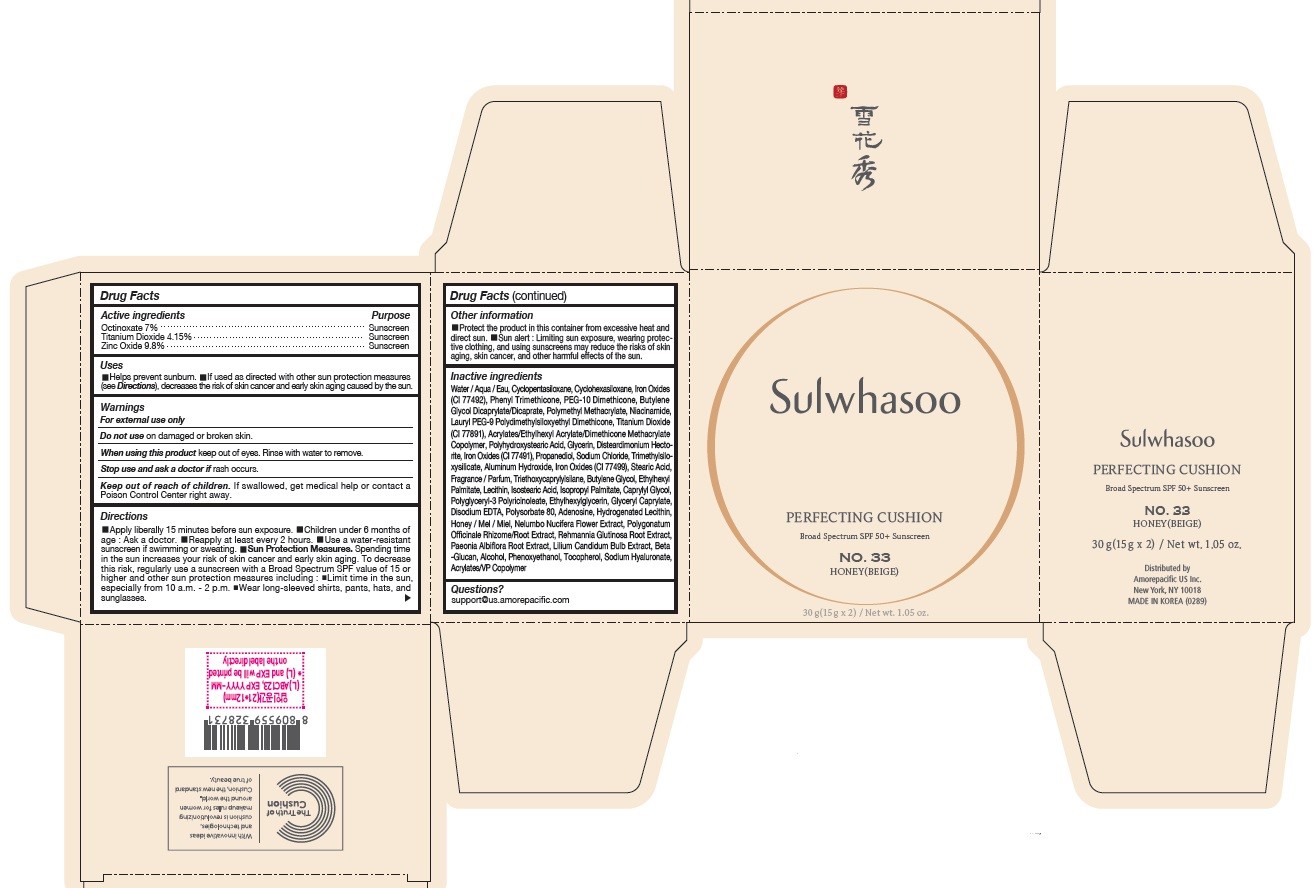
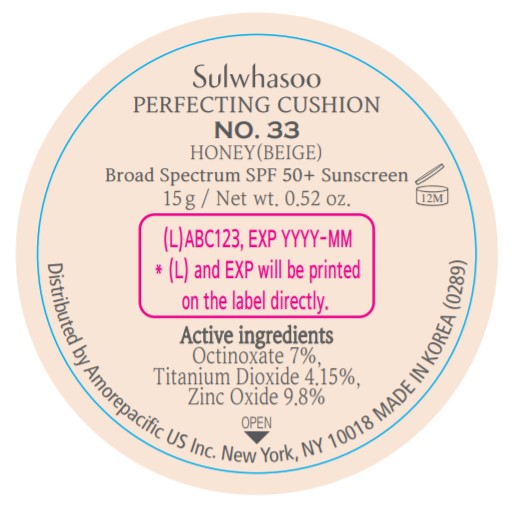
SULWHASOO PERFECTING CUSHION NO. 33- octinoxate, titanium dioxide, and zinc oxide lotion
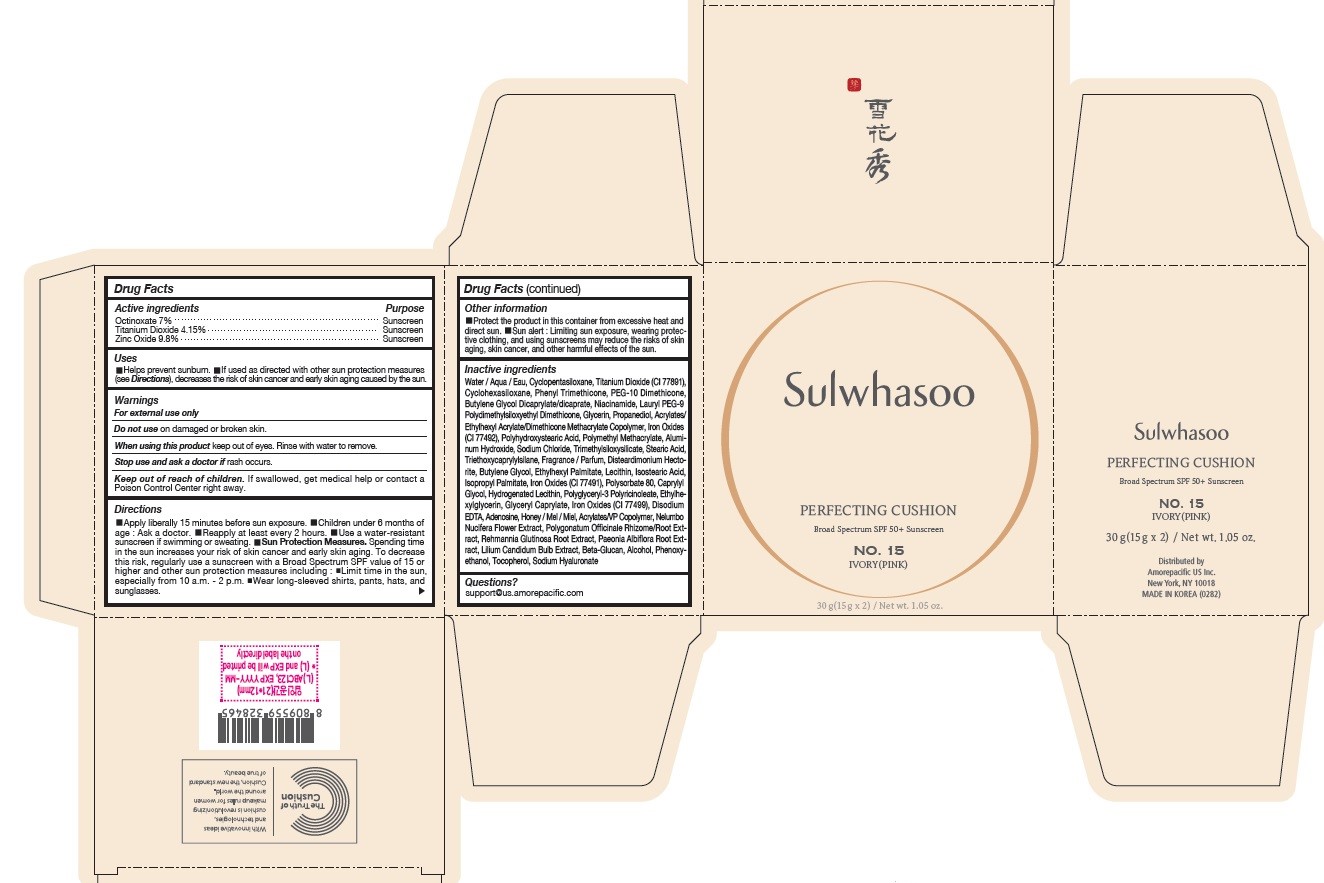
SULWHASOO PERFECTING CUSHION NO. 15- octinoxate, titanium dioxide, and zinc oxide lotion
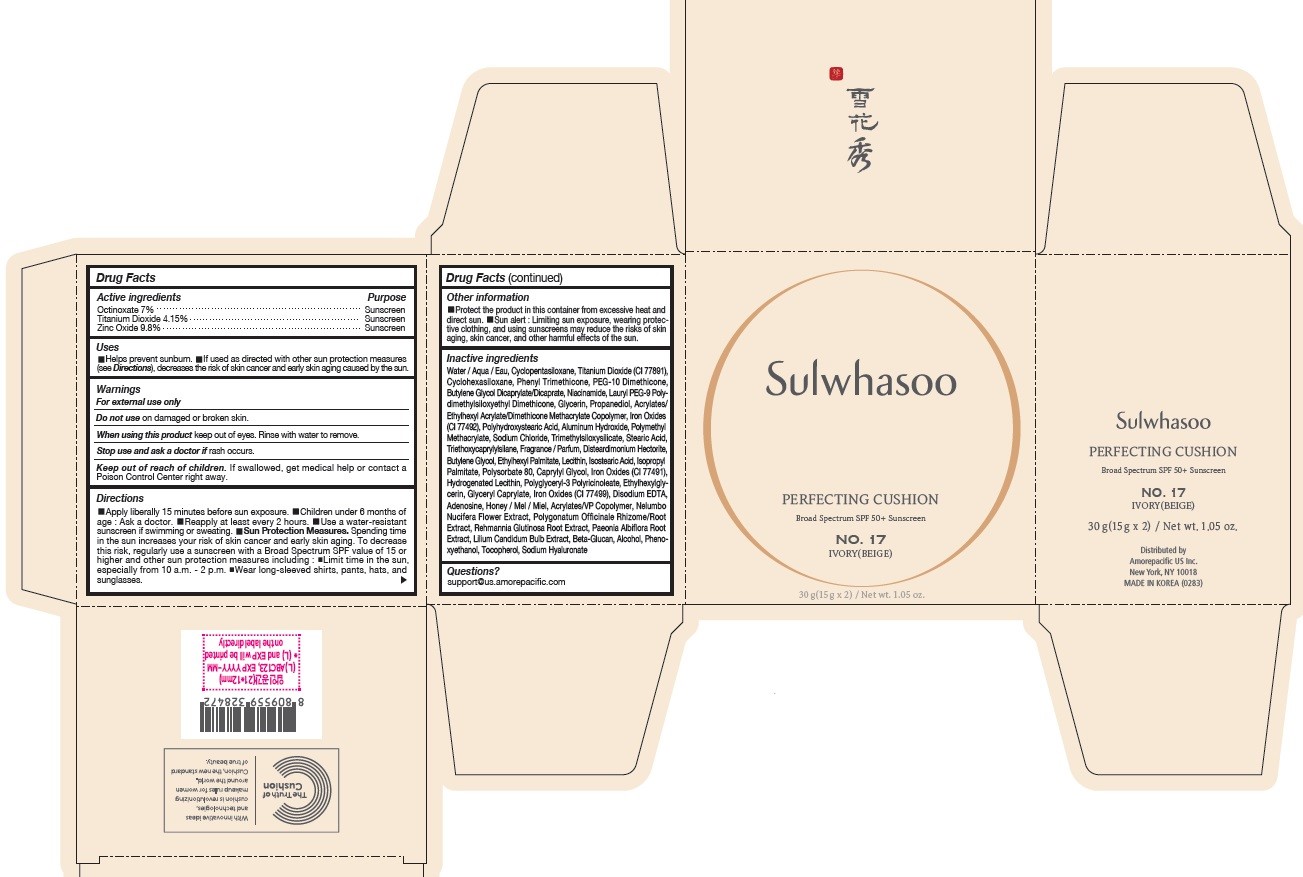
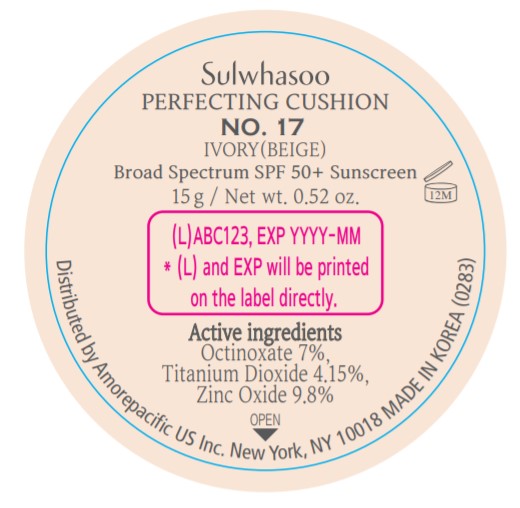
SULWHASOO PERFECTING CUSHION NO. 17- octinoxate, titanium dioxide, and zinc oxide lotion
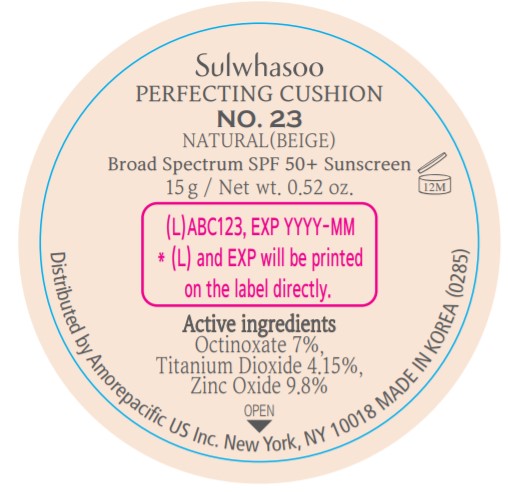
SULWHASOO PERFECTING CUSHION NO. 23- octinoxate, titanium dioxide, and zinc oxide lotion
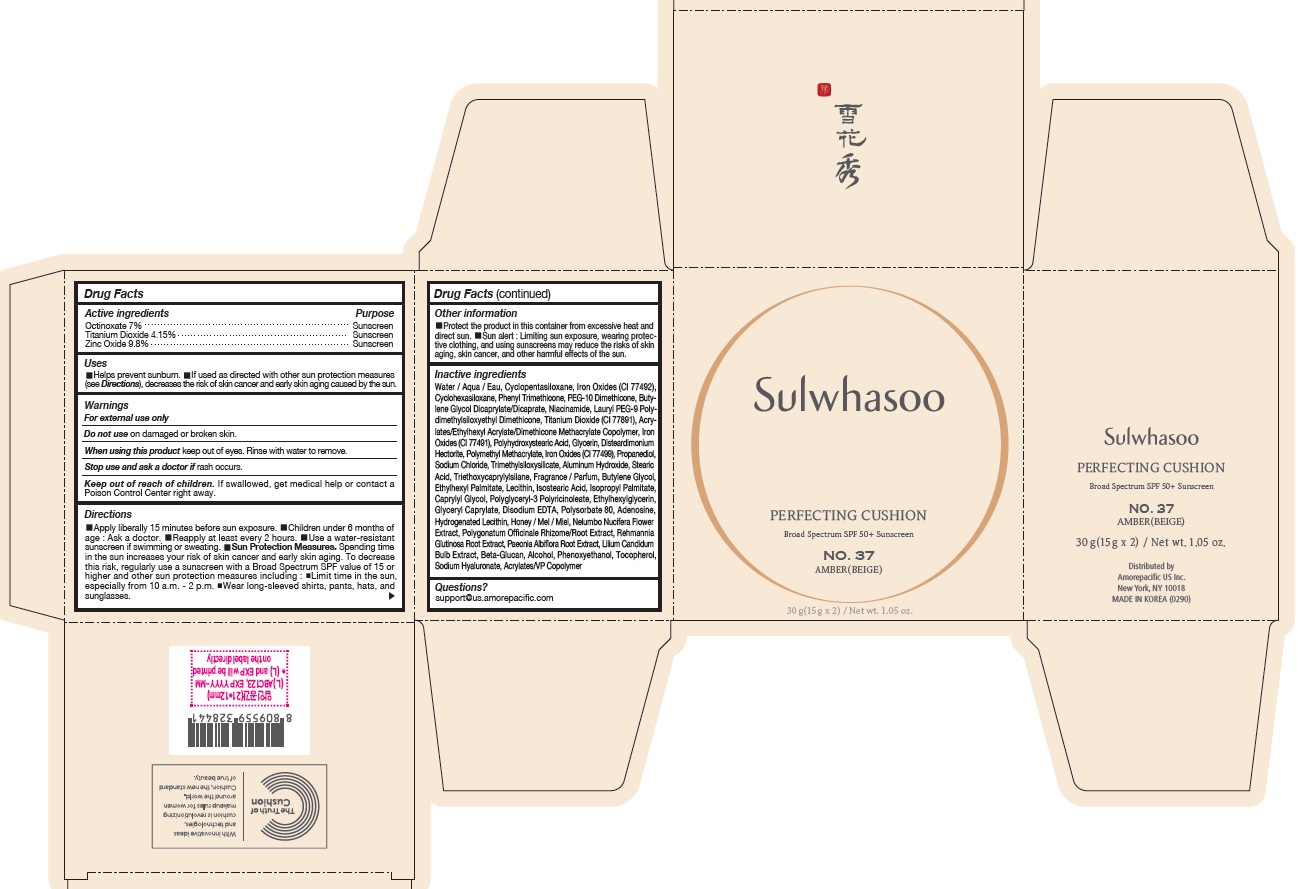
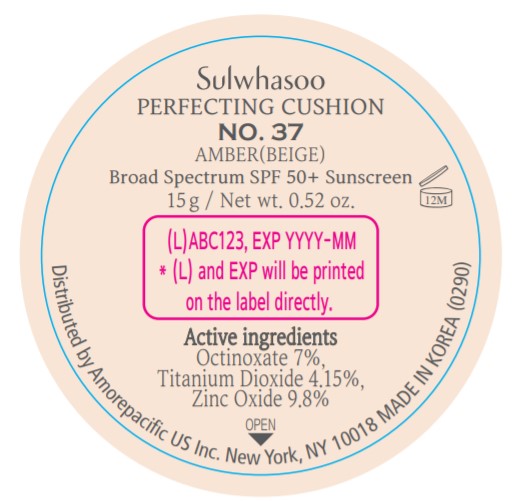
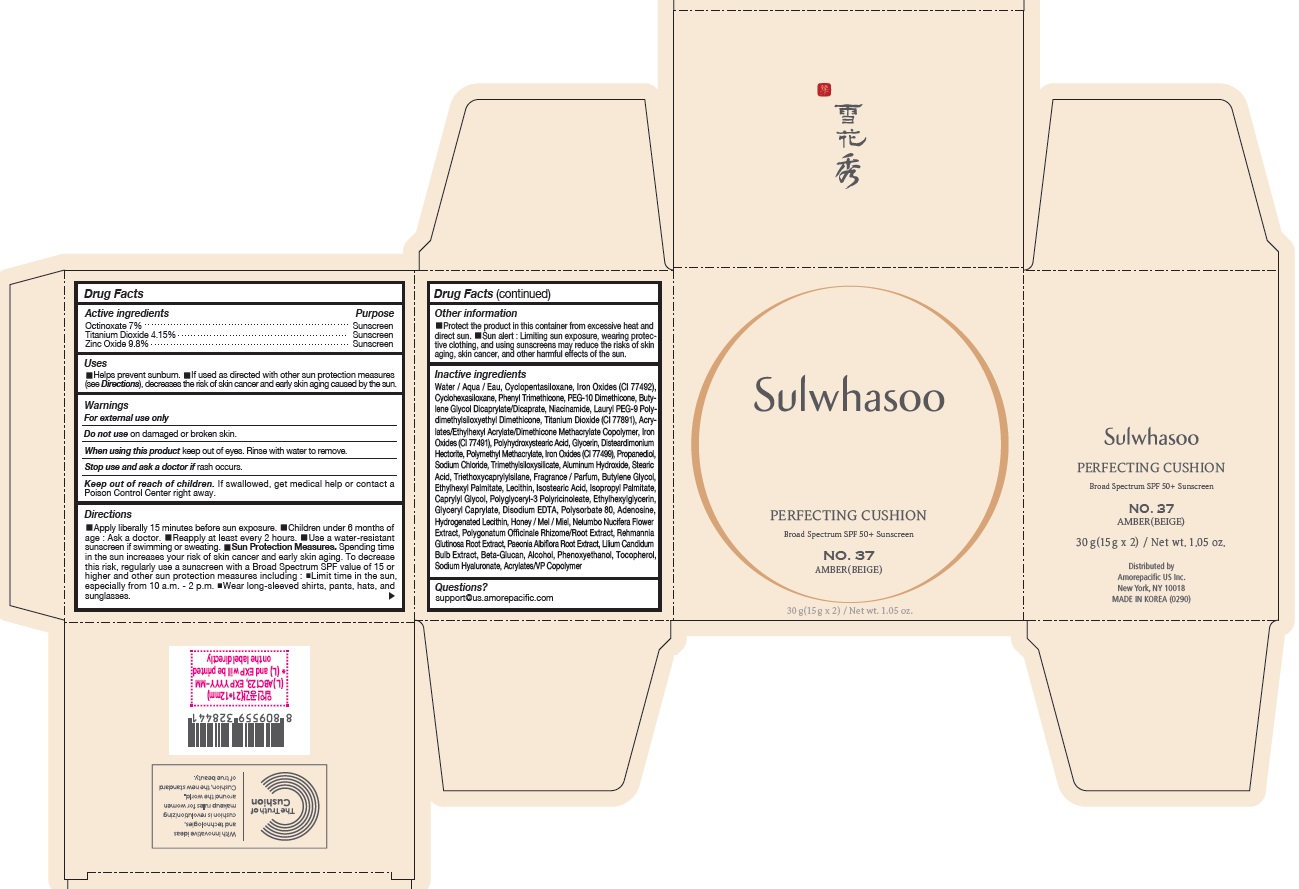
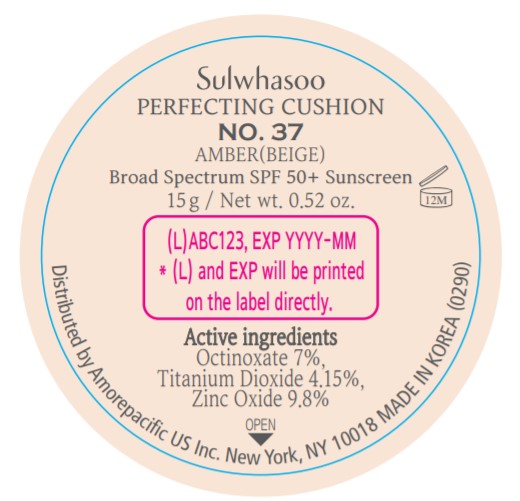
SULWHASOO PERFECTING CUSHION NO. 37- octinoxate, titanium dioxide, and zinc oxide lotion
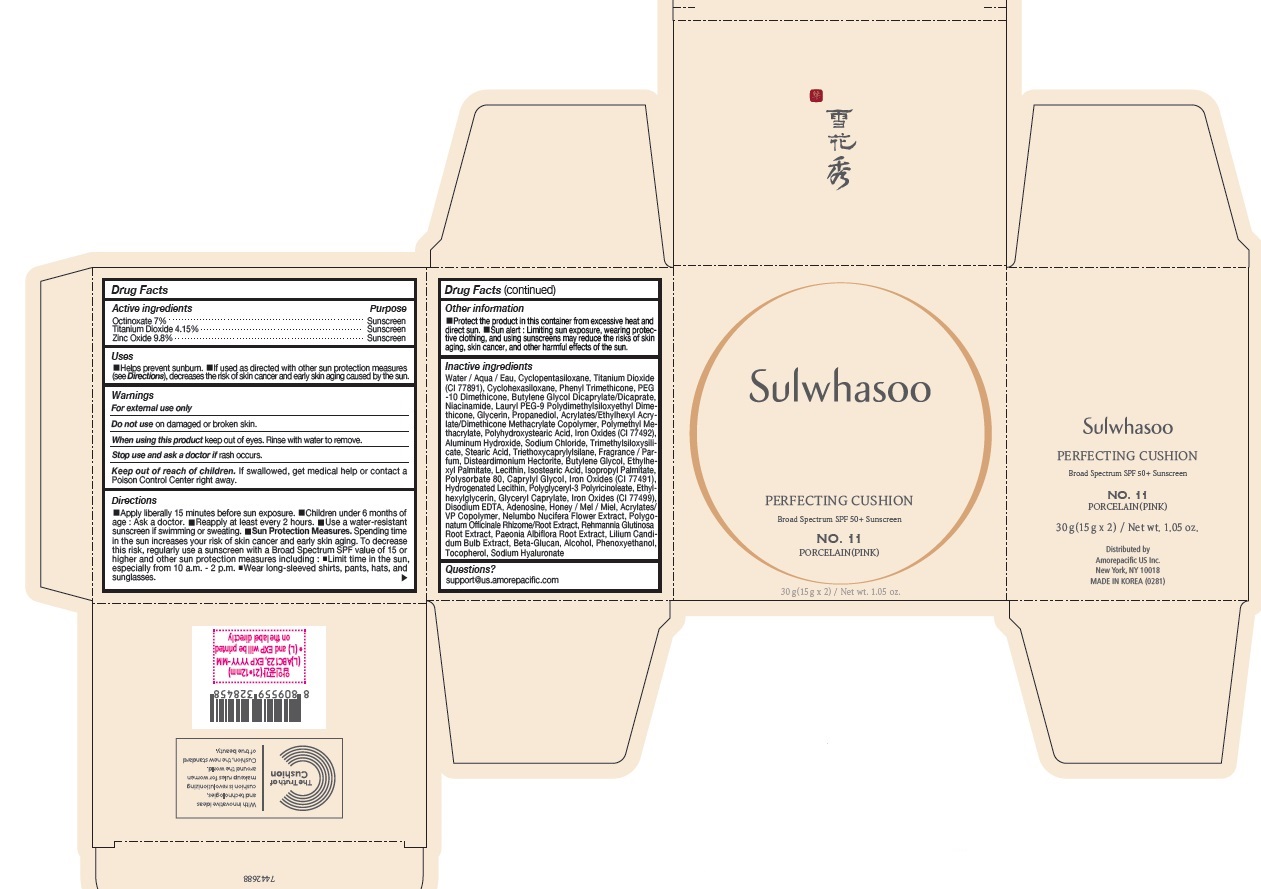
SULWHASOO PERFECTING CUSHION NO. 11- octinoxate, titanium dioxide, and zinc oxide lotion
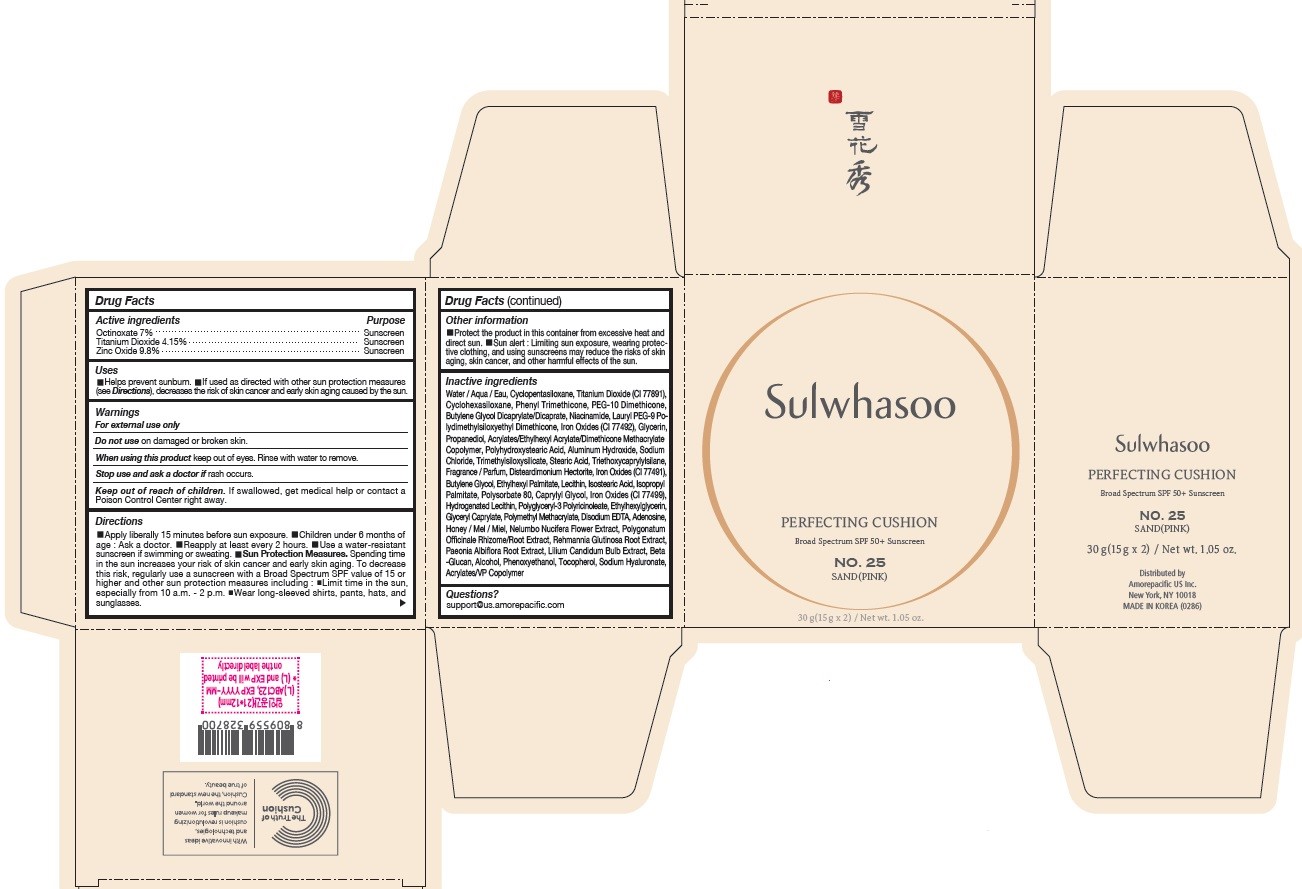
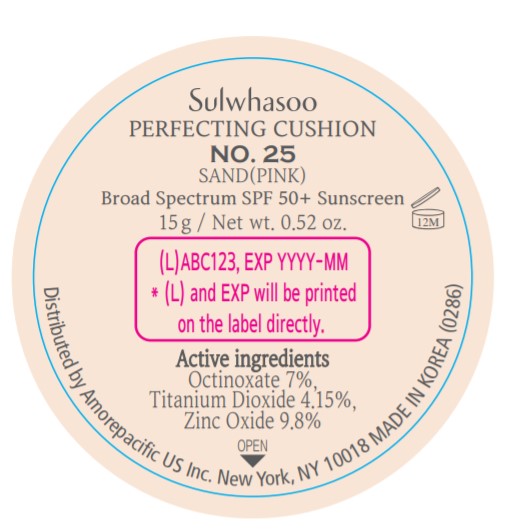
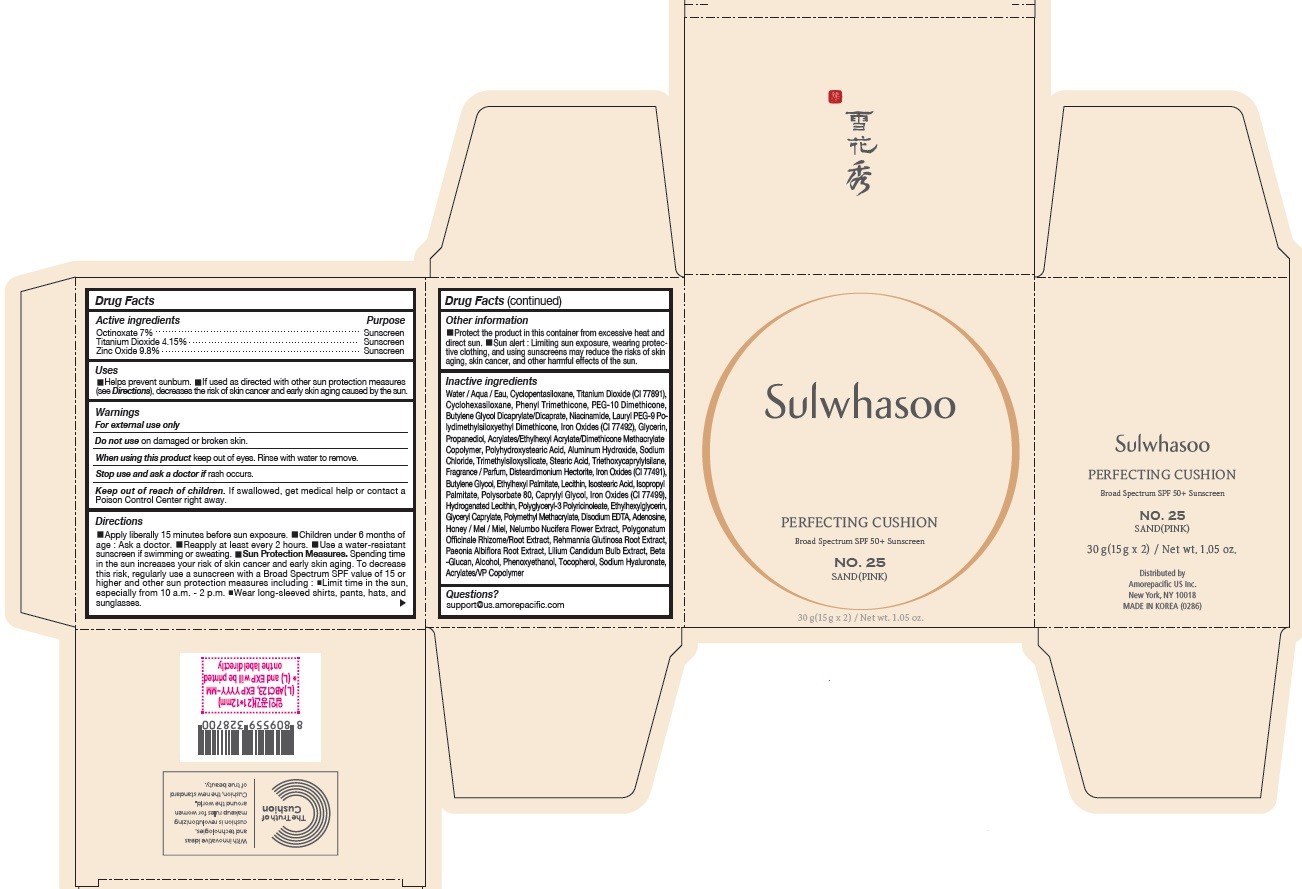
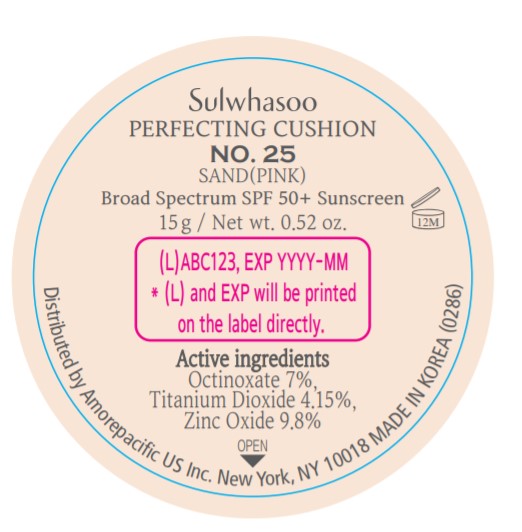
SULWHASOO PERFECTING CUSHION NO. 25- octinoxate, titanium dioxide, and zinc oxide lotion
-
NDC Code(s):
43419-074-31,
43419-075-31,
43419-076-31,
43419-077-31, view more43419-077-39, 43419-078-31, 43419-078-39, 43419-079-31, 43419-079-39, 43419-080-31, 43419-081-31, 43419-082-31, 43419-083-31
- Packager: Amorepacific Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
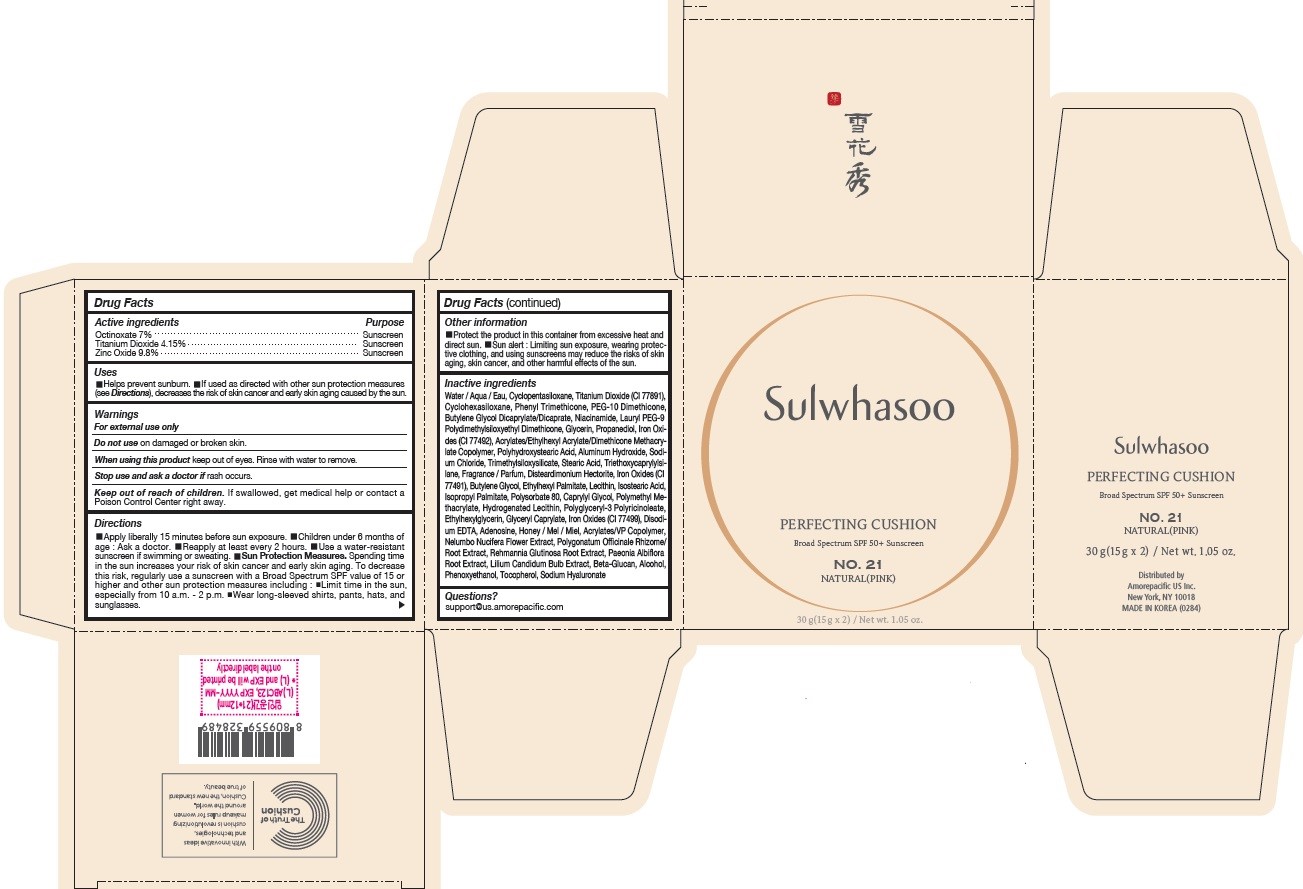
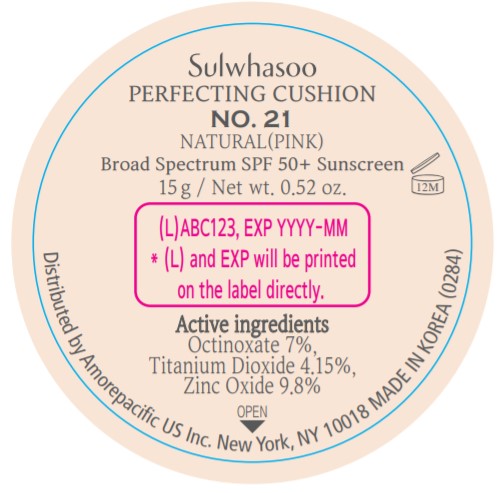
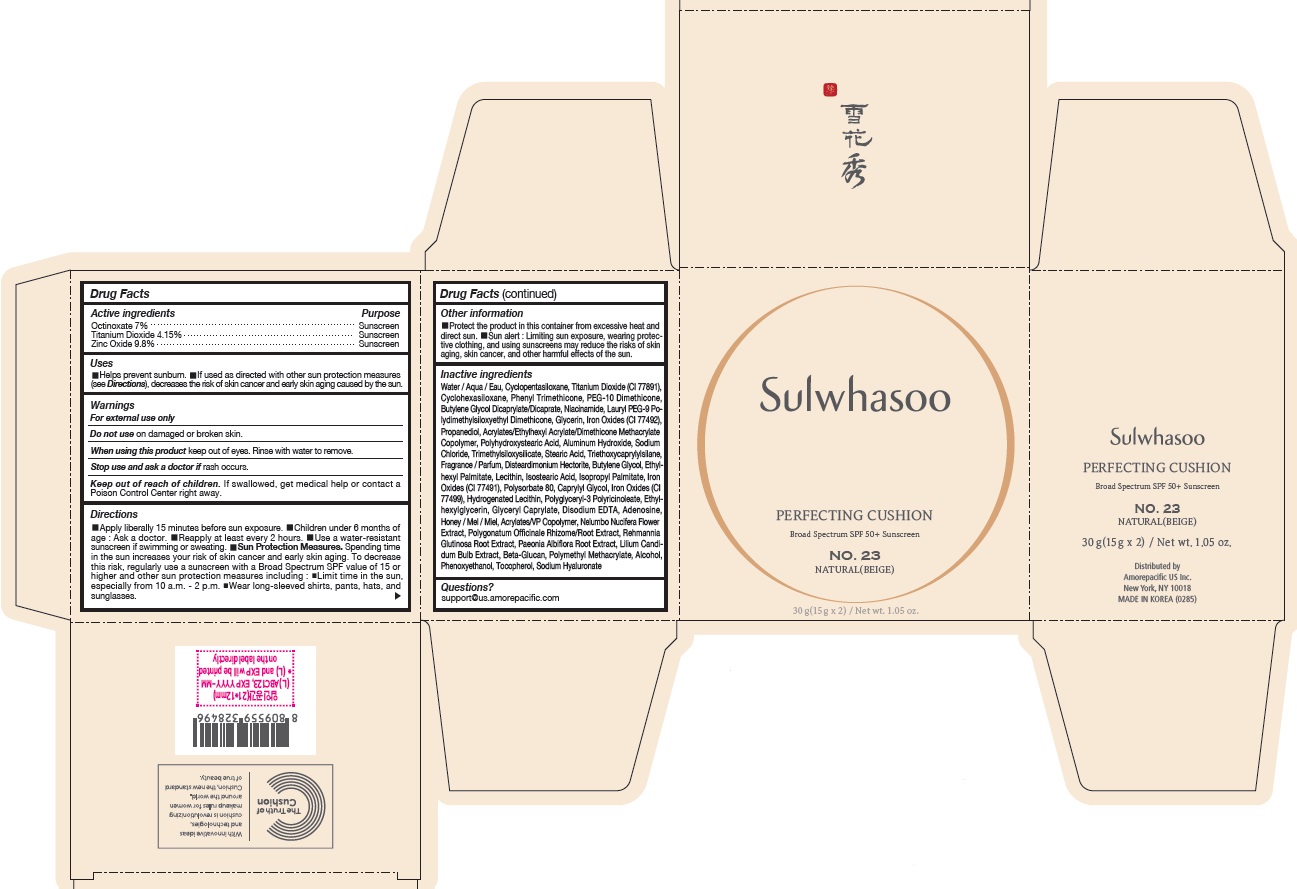
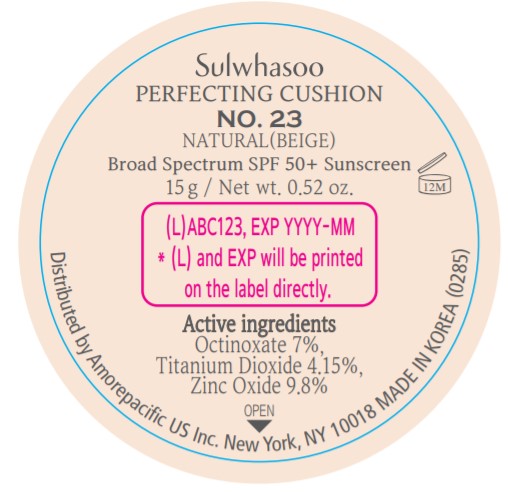
- ACTIVE INGREDIENTS W/W
- PURPOSE
-
USES
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions ), decreases the risk of skin cancer and early skin aging caused by the sun
- WARNINGS
-
DIRECTIONS
- Apply liberally 15 minutes before sun exposure
- Children under 6 months of age : Ask a doctor.
- Reapply at least every 2 hours.
- Use a water-resistant sunscreen if swimming or sweating.
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including :
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
-
INACTIVE INGREDIENTS
Water / Aqua / Eau, Cyclopentasiloxane, Titanium Dioxide (CI 77891), Cyclohexasiloxane, Phenyl Trimethicone, PEG-10 Dimethicone, Butylene Glycol Dicaprylate/Dicaprate, Niacinamide, Lauryl PEG-9 Polydimethylsiloxyethyl Dimethicone, Glycerin, Propanediol, Acrylates/Ethylhexyl Acrylate/Dimethicone Methacrylate Copolymer, Polymethyl Methacrylate, Polyhydroxystearic Acid, Iron Oxides (CI 77492), Aluminum Hydroxide, Sodium Chloride, Trimethylsiloxysilicate, Stearic Acid, Triethoxycaprylylsilane, Fragrance / Parfum, Disteardimonium Hectorite, Butylene Glycol, Ethylhexyl Palmitate, Lecithin, Isostearic Acid, Isopropyl Palmitate, Polysorbate 80, Caprylyl Glycol, Iron Oxides (CI 77491), Hydrogenated Lecithin, Polyglyceryl-3 Polyricinoleate, Ethylhexylglycerin, Glyceryl Caprylate, Iron Oxides (CI 77499), Disodium EDTA, Adenosine, Honey / Mel / Miel, Acrylates/VP Copolymer, Nelumbo Nucifera Flower Extract, Polygonatum Officinale Rhizome/Root Extract, Rehmannia Glutinosa Root Extract, Paeonia Albiflora Root Extract, Lilium Candidum Bulb Extract, Beta-Glucan, Alcohol, Phenoxyethanol, Tocopherol, Sodium Hyaluronate
- OTHER INFORMATION
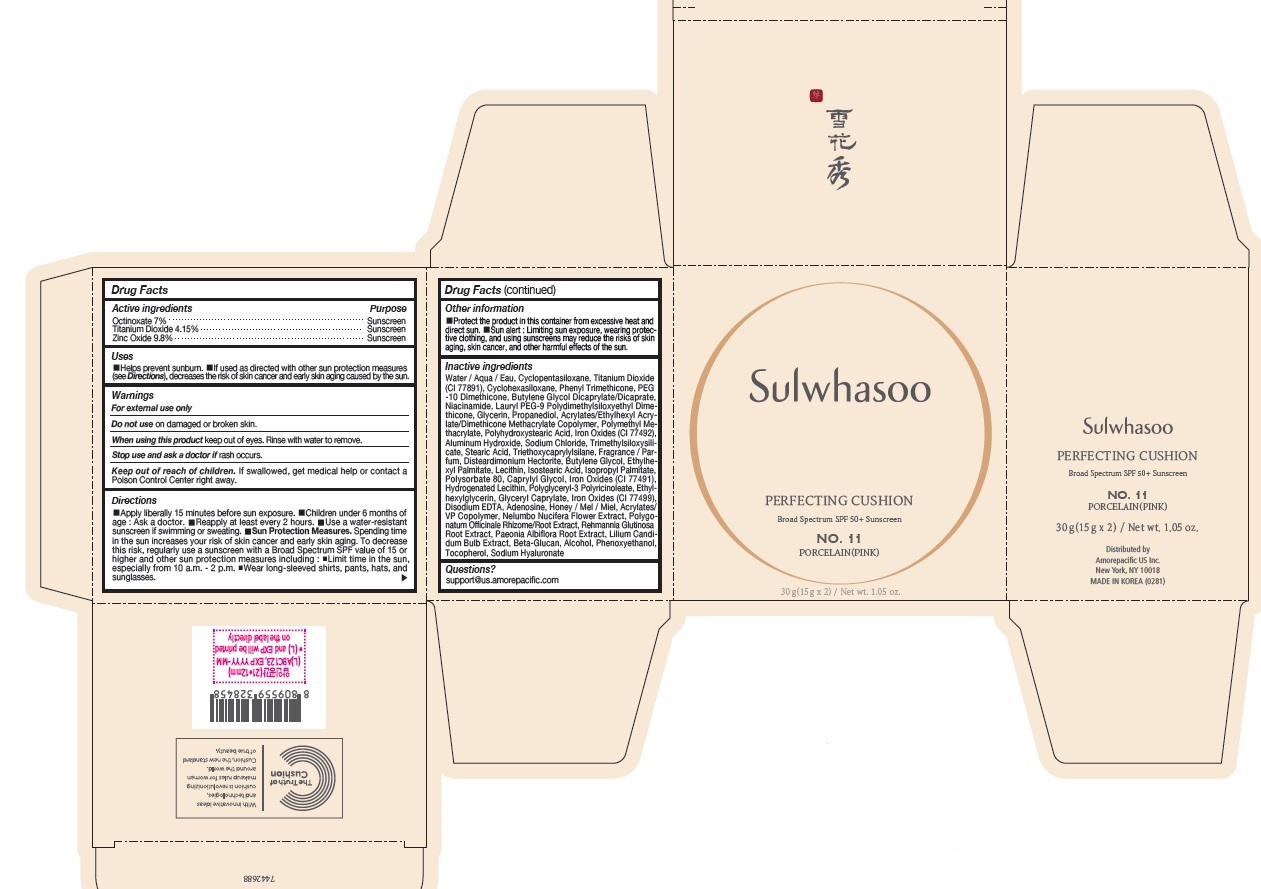
- PRINCIPAL DISPLAY PANEL - NO. 11 PORCELAIN(PINK)
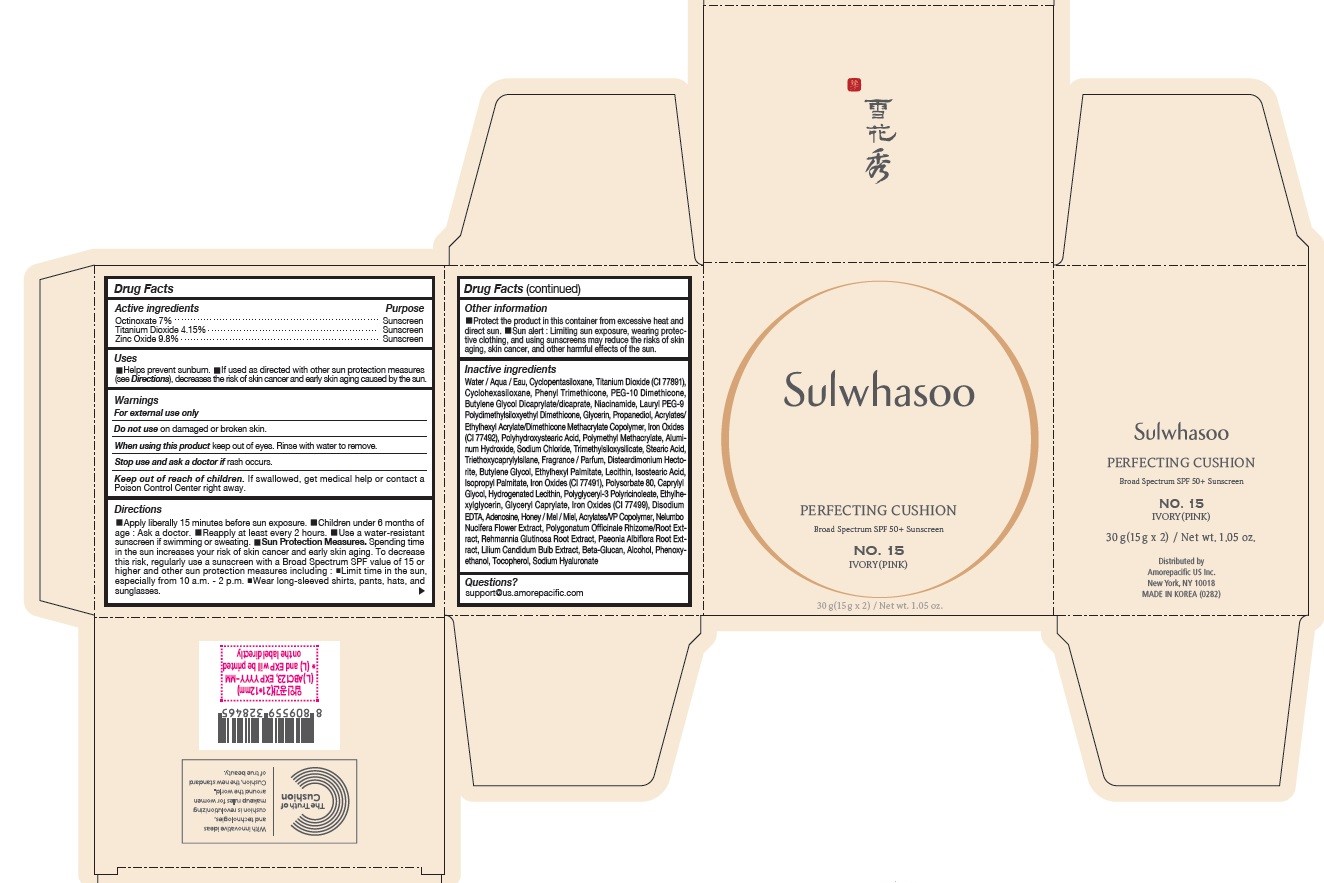
- PRINCIPAL DISPLAY PANEL - NO. 15 IVORY(PINK)
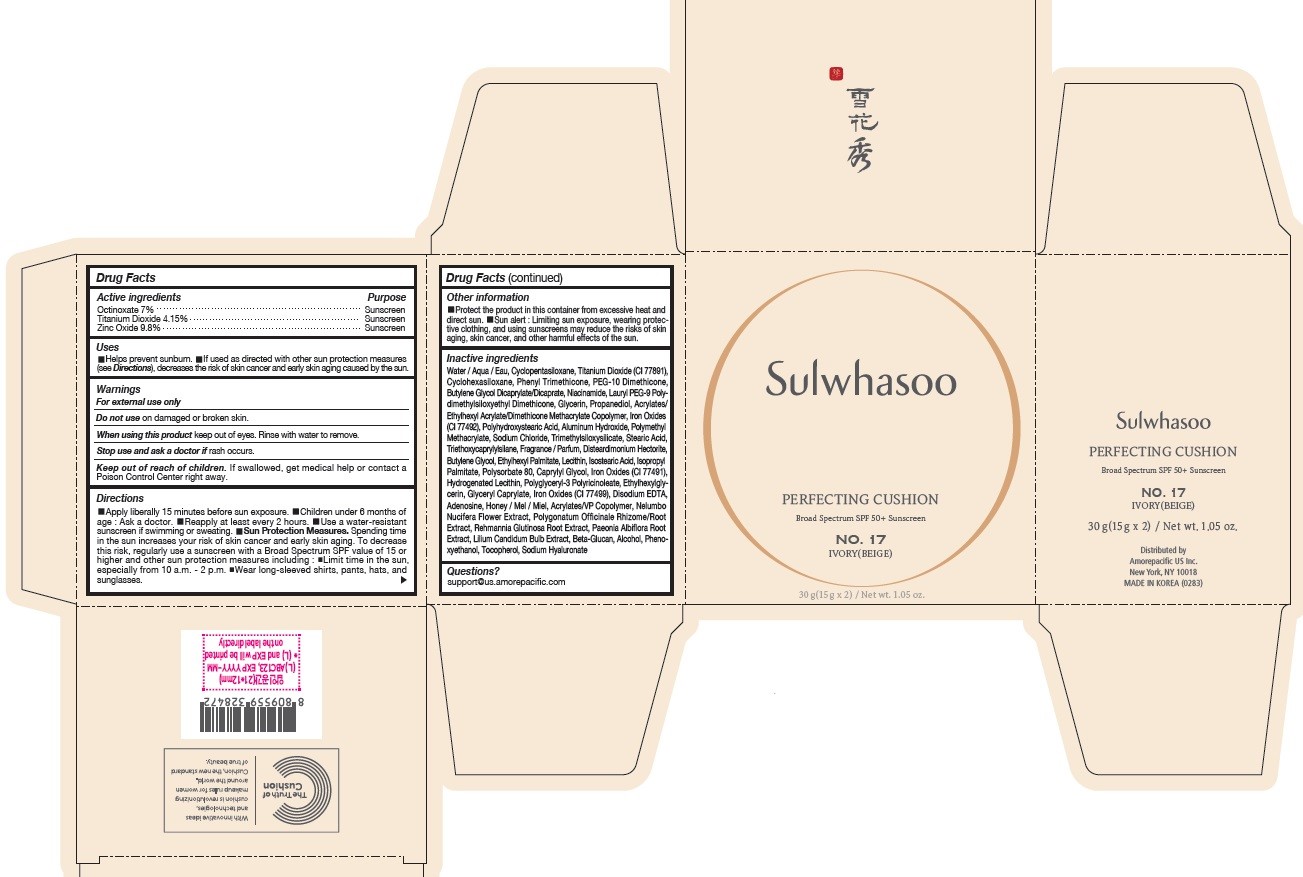
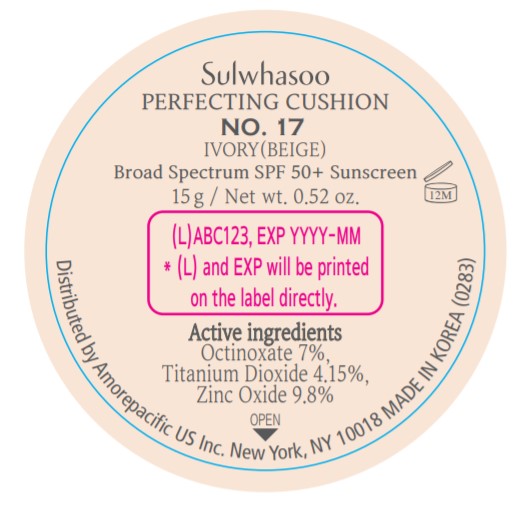
- PRINCIPAL DISPLAY PANEL - NO. 17 IVORY(BEIGE)
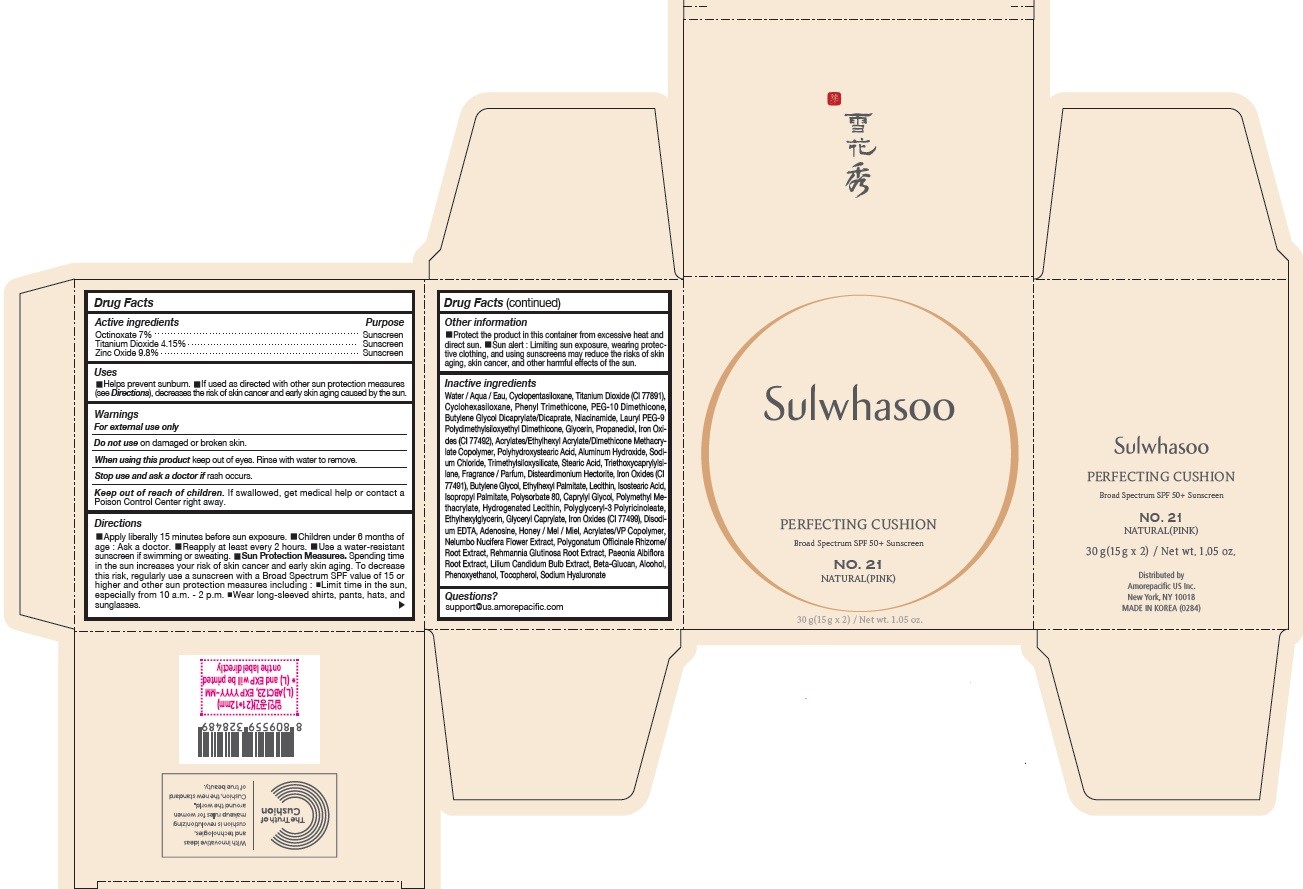
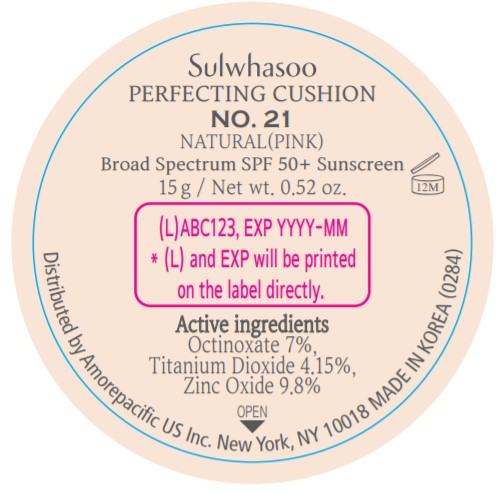
- PRINCIPAL DISPLAY PANEL - NO. 21 NATURAL(PINK)
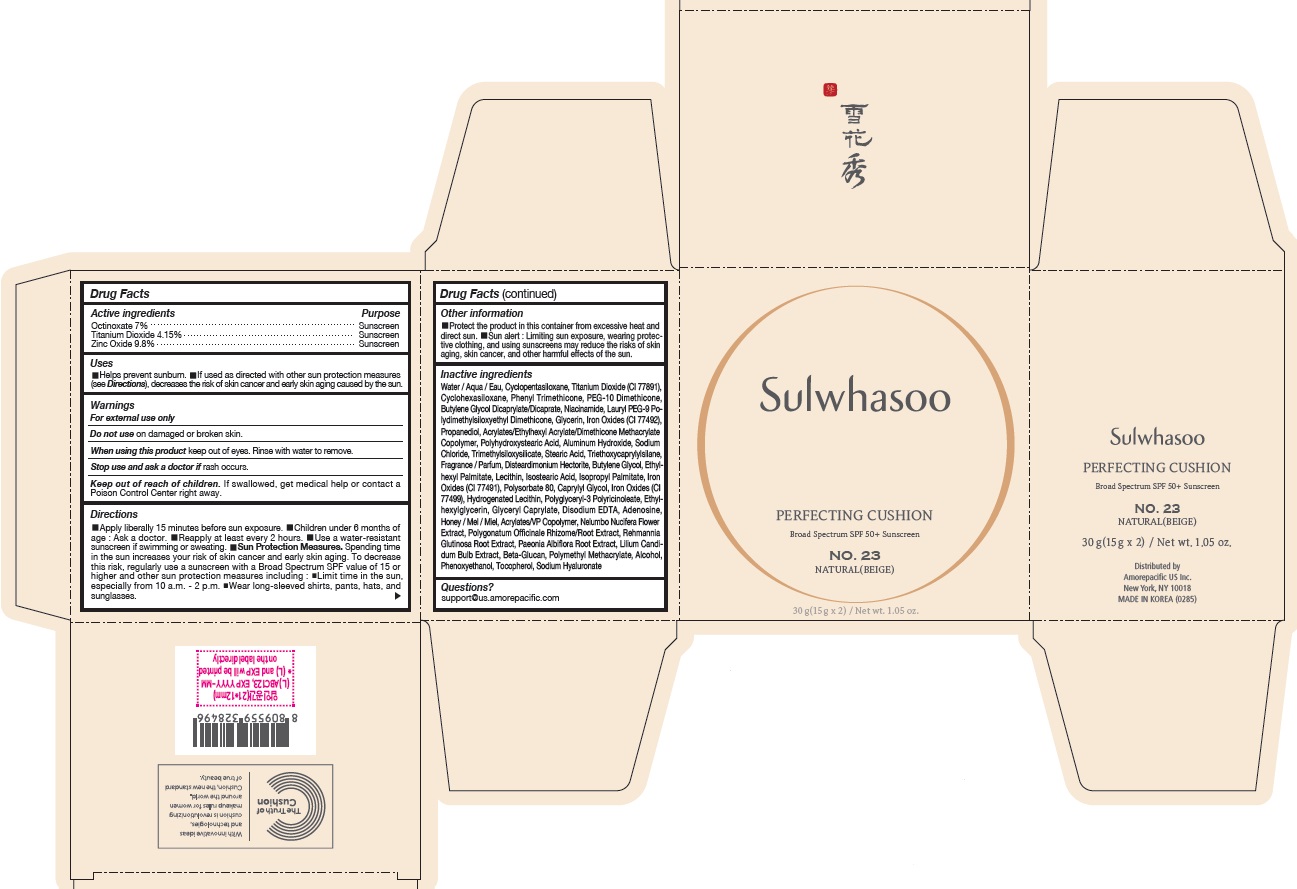
- PRINCIPAL DISPLAY PANEL - NO. 23 NATURAL(BEIGE)
- PRINCIPAL DISPLAY PANEL - NO. 25 SAND(PINK)
- PRINCIPAL DISPLAY PANEL - NO. 27 SAND(BEIGE)
- PRINCIPAL DISPLAY PANEL - NO. 31 HONEY(PINK)
- PRINCIPAL DISPLAY PANEL - NO. 33 HONEY(BEIGE)
- PRINCIPAL DISPLAY PANEL - NO. 37 AMBER(BEIGE)
-
INGREDIENTS AND APPEARANCE
SULWHASOO PERFECTING CUSHION NO. 21
octinoxate, titanium dioxide, and zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43419-077 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.1 g in 30 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.245 g in 30 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 2.94 g in 30 g Inactive Ingredients Ingredient Name Strength HYALURONATE SODIUM (UNII: YSE9PPT4TH) TOCOPHEROL (UNII: R0ZB2556P8) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) NIACINAMIDE (UNII: 25X51I8RD4) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) GLYCERIN (UNII: PDC6A3C0OX) PROPANEDIOL (UNII: 5965N8W85T) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRIMETHYLSILOXYSILICATE (M/Q 1.0-1.2) (UNII: 78GX033D7I) STEARIC ACID (UNII: 4ELV7Z65AP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOSTEARIC ACID (UNII: X33R8U0062) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) POLYSORBATE 80 (UNII: 6OZP39ZG8H) CAPRYLYL GLYCOL (UNII: 00YIU5438U) FERRIC OXIDE RED (UNII: 1K09F3G675) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) FERROSOFERRIC OXIDE (UNII: XM0M87F357) EDETATE DISODIUM (UNII: 7FLD91C86K) ADENOSINE (UNII: K72T3FS567) HONEY (UNII: Y9H1V576FH) NELUMBO NUCIFERA FLOWER (UNII: 61W322NLDV) POLYGONATUM ODORATUM ROOT (UNII: KPF03O64AZ) LILIUM CANDIDUM BULB (UNII: AHG15J8AM0) .BETA.-D-GLUCOPYRANOSE (UNII: J4R00M814D) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43419-077-31 2 in 1 CARTON 07/15/2019 1 15 g in 1 CONTAINER; Type 0: Not a Combination Product 2 NDC:43419-077-39 2 in 1 CARTON 12/09/2019 12/02/2021 2 15 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 07/15/2019 SULWHASOO PERFECTING CUSHION NO. 27
octinoxate, titanium dioxide, and zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43419-080 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.1 g in 30 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.245 g in 30 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 2.94 g in 30 g Inactive Ingredients Ingredient Name Strength HYALURONATE SODIUM (UNII: YSE9PPT4TH) TOCOPHEROL (UNII: R0ZB2556P8) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) NIACINAMIDE (UNII: 25X51I8RD4) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) GLYCERIN (UNII: PDC6A3C0OX) PROPANEDIOL (UNII: 5965N8W85T) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRIMETHYLSILOXYSILICATE (M/Q 1.0-1.2) (UNII: 78GX033D7I) STEARIC ACID (UNII: 4ELV7Z65AP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ETHYLHEXYL PALMITATE (UNII: 2865993309) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) ISOSTEARIC ACID (UNII: X33R8U0062) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) POLYSORBATE 80 (UNII: 6OZP39ZG8H) CAPRYLYL GLYCOL (UNII: 00YIU5438U) FERRIC OXIDE RED (UNII: 1K09F3G675) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) FERROSOFERRIC OXIDE (UNII: XM0M87F357) EDETATE DISODIUM (UNII: 7FLD91C86K) ADENOSINE (UNII: K72T3FS567) HONEY (UNII: Y9H1V576FH) NELUMBO NUCIFERA FLOWER (UNII: 61W322NLDV) POLYGONATUM ODORATUM ROOT (UNII: KPF03O64AZ) LILIUM CANDIDUM BULB (UNII: AHG15J8AM0) .BETA.-D-GLUCOPYRANOSE (UNII: J4R00M814D) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43419-080-31 2 in 1 CARTON 07/15/2019 10/01/2022 1 15 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 07/15/2019 10/01/2022 SULWHASOO PERFECTING CUSHION NO. 31
octinoxate, titanium dioxide, and zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43419-081 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.1 g in 30 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.245 g in 30 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 2.94 g in 30 g Inactive Ingredients Ingredient Name Strength ETHYLHEXYL PALMITATE (UNII: 2865993309) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) ISOSTEARIC ACID (UNII: X33R8U0062) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) POLYSORBATE 80 (UNII: 6OZP39ZG8H) CAPRYLYL GLYCOL (UNII: 00YIU5438U) FERRIC OXIDE RED (UNII: 1K09F3G675) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) FERROSOFERRIC OXIDE (UNII: XM0M87F357) EDETATE DISODIUM (UNII: 7FLD91C86K) ADENOSINE (UNII: K72T3FS567) HONEY (UNII: Y9H1V576FH) NELUMBO NUCIFERA FLOWER (UNII: 61W322NLDV) POLYGONATUM ODORATUM ROOT (UNII: KPF03O64AZ) LILIUM CANDIDUM BULB (UNII: AHG15J8AM0) .BETA.-D-GLUCOPYRANOSE (UNII: J4R00M814D) ALCOHOL (UNII: 3K9958V90M) HYALURONATE SODIUM (UNII: YSE9PPT4TH) TOCOPHEROL (UNII: R0ZB2556P8) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) NIACINAMIDE (UNII: 25X51I8RD4) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) GLYCERIN (UNII: PDC6A3C0OX) PROPANEDIOL (UNII: 5965N8W85T) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRIMETHYLSILOXYSILICATE (M/Q 1.0-1.2) (UNII: 78GX033D7I) STEARIC ACID (UNII: 4ELV7Z65AP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43419-081-31 2 in 1 CARTON 07/15/2019 10/01/2022 1 15 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 07/15/2019 10/01/2022 SULWHASOO PERFECTING CUSHION NO. 33
octinoxate, titanium dioxide, and zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43419-082 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.1 g in 30 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.245 g in 30 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 2.94 g in 30 g Inactive Ingredients Ingredient Name Strength HYALURONATE SODIUM (UNII: YSE9PPT4TH) TOCOPHEROL (UNII: R0ZB2556P8) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) NIACINAMIDE (UNII: 25X51I8RD4) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) GLYCERIN (UNII: PDC6A3C0OX) PROPANEDIOL (UNII: 5965N8W85T) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRIMETHYLSILOXYSILICATE (M/Q 1.0-1.2) (UNII: 78GX033D7I) STEARIC ACID (UNII: 4ELV7Z65AP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOSTEARIC ACID (UNII: X33R8U0062) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) POLYSORBATE 80 (UNII: 6OZP39ZG8H) CAPRYLYL GLYCOL (UNII: 00YIU5438U) FERRIC OXIDE RED (UNII: 1K09F3G675) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) FERROSOFERRIC OXIDE (UNII: XM0M87F357) EDETATE DISODIUM (UNII: 7FLD91C86K) ADENOSINE (UNII: K72T3FS567) HONEY (UNII: Y9H1V576FH) NELUMBO NUCIFERA FLOWER (UNII: 61W322NLDV) POLYGONATUM ODORATUM ROOT (UNII: KPF03O64AZ) LILIUM CANDIDUM BULB (UNII: AHG15J8AM0) .BETA.-D-GLUCOPYRANOSE (UNII: J4R00M814D) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43419-082-31 2 in 1 CARTON 07/15/2019 10/31/2021 1 15 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 07/15/2019 10/31/2021 SULWHASOO PERFECTING CUSHION NO. 15
octinoxate, titanium dioxide, and zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43419-075 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.1 g in 30 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.245 g in 30 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 2.94 g in 30 g Inactive Ingredients Ingredient Name Strength BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOSTEARIC ACID (UNII: X33R8U0062) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) POLYSORBATE 80 (UNII: 6OZP39ZG8H) CAPRYLYL GLYCOL (UNII: 00YIU5438U) FERRIC OXIDE RED (UNII: 1K09F3G675) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) FERROSOFERRIC OXIDE (UNII: XM0M87F357) EDETATE DISODIUM (UNII: 7FLD91C86K) ADENOSINE (UNII: K72T3FS567) HONEY (UNII: Y9H1V576FH) NELUMBO NUCIFERA FLOWER (UNII: 61W322NLDV) POLYGONATUM ODORATUM ROOT (UNII: KPF03O64AZ) LILIUM CANDIDUM BULB (UNII: AHG15J8AM0) .BETA.-D-GLUCOPYRANOSE (UNII: J4R00M814D) ALCOHOL (UNII: 3K9958V90M) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) HYALURONATE SODIUM (UNII: YSE9PPT4TH) TOCOPHEROL (UNII: R0ZB2556P8) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) NIACINAMIDE (UNII: 25X51I8RD4) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) GLYCERIN (UNII: PDC6A3C0OX) PROPANEDIOL (UNII: 5965N8W85T) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRIMETHYLSILOXYSILICATE (M/Q 1.0-1.2) (UNII: 78GX033D7I) STEARIC ACID (UNII: 4ELV7Z65AP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43419-075-31 2 in 1 CARTON 07/15/2019 1 15 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 07/15/2019 SULWHASOO PERFECTING CUSHION NO. 17
octinoxate, titanium dioxide, and zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43419-076 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.1 g in 30 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.245 g in 30 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 2.94 g in 30 g Inactive Ingredients Ingredient Name Strength HYALURONATE SODIUM (UNII: YSE9PPT4TH) TOCOPHEROL (UNII: R0ZB2556P8) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) NIACINAMIDE (UNII: 25X51I8RD4) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) GLYCERIN (UNII: PDC6A3C0OX) PROPANEDIOL (UNII: 5965N8W85T) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRIMETHYLSILOXYSILICATE (M/Q 1.0-1.2) (UNII: 78GX033D7I) STEARIC ACID (UNII: 4ELV7Z65AP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ETHYLHEXYL PALMITATE (UNII: 2865993309) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) ISOSTEARIC ACID (UNII: X33R8U0062) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) POLYSORBATE 80 (UNII: 6OZP39ZG8H) CAPRYLYL GLYCOL (UNII: 00YIU5438U) FERRIC OXIDE RED (UNII: 1K09F3G675) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) FERROSOFERRIC OXIDE (UNII: XM0M87F357) EDETATE DISODIUM (UNII: 7FLD91C86K) ADENOSINE (UNII: K72T3FS567) HONEY (UNII: Y9H1V576FH) NELUMBO NUCIFERA FLOWER (UNII: 61W322NLDV) POLYGONATUM ODORATUM ROOT (UNII: KPF03O64AZ) LILIUM CANDIDUM BULB (UNII: AHG15J8AM0) .BETA.-D-GLUCOPYRANOSE (UNII: J4R00M814D) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43419-076-31 2 in 1 CARTON 07/15/2019 12/25/2021 1 15 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 07/15/2019 12/25/2021 SULWHASOO PERFECTING CUSHION NO. 23
octinoxate, titanium dioxide, and zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43419-078 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.1 g in 30 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.245 g in 30 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 2.94 g in 30 g Inactive Ingredients Ingredient Name Strength HYALURONATE SODIUM (UNII: YSE9PPT4TH) TOCOPHEROL (UNII: R0ZB2556P8) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) NIACINAMIDE (UNII: 25X51I8RD4) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) GLYCERIN (UNII: PDC6A3C0OX) PROPANEDIOL (UNII: 5965N8W85T) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRIMETHYLSILOXYSILICATE (M/Q 1.0-1.2) (UNII: 78GX033D7I) STEARIC ACID (UNII: 4ELV7Z65AP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ETHYLHEXYL PALMITATE (UNII: 2865993309) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) ISOSTEARIC ACID (UNII: X33R8U0062) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) POLYSORBATE 80 (UNII: 6OZP39ZG8H) CAPRYLYL GLYCOL (UNII: 00YIU5438U) FERRIC OXIDE RED (UNII: 1K09F3G675) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) FERROSOFERRIC OXIDE (UNII: XM0M87F357) EDETATE DISODIUM (UNII: 7FLD91C86K) ADENOSINE (UNII: K72T3FS567) HONEY (UNII: Y9H1V576FH) NELUMBO NUCIFERA FLOWER (UNII: 61W322NLDV) POLYGONATUM ODORATUM ROOT (UNII: KPF03O64AZ) LILIUM CANDIDUM BULB (UNII: AHG15J8AM0) .BETA.-D-GLUCOPYRANOSE (UNII: J4R00M814D) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43419-078-31 2 in 1 CARTON 07/15/2019 1 15 g in 1 CONTAINER; Type 0: Not a Combination Product 2 NDC:43419-078-39 2 in 1 CARTON 12/09/2019 12/03/2021 2 15 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 07/15/2019 SULWHASOO PERFECTING CUSHION NO. 37
octinoxate, titanium dioxide, and zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43419-083 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.1 g in 30 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.245 g in 30 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 2.94 g in 30 g Inactive Ingredients Ingredient Name Strength HYALURONATE SODIUM (UNII: YSE9PPT4TH) TOCOPHEROL (UNII: R0ZB2556P8) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) NIACINAMIDE (UNII: 25X51I8RD4) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) GLYCERIN (UNII: PDC6A3C0OX) PROPANEDIOL (UNII: 5965N8W85T) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRIMETHYLSILOXYSILICATE (M/Q 1.0-1.2) (UNII: 78GX033D7I) STEARIC ACID (UNII: 4ELV7Z65AP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOSTEARIC ACID (UNII: X33R8U0062) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) POLYSORBATE 80 (UNII: 6OZP39ZG8H) CAPRYLYL GLYCOL (UNII: 00YIU5438U) FERRIC OXIDE RED (UNII: 1K09F3G675) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) FERROSOFERRIC OXIDE (UNII: XM0M87F357) EDETATE DISODIUM (UNII: 7FLD91C86K) ADENOSINE (UNII: K72T3FS567) HONEY (UNII: Y9H1V576FH) NELUMBO NUCIFERA FLOWER (UNII: 61W322NLDV) POLYGONATUM ODORATUM ROOT (UNII: KPF03O64AZ) LILIUM CANDIDUM BULB (UNII: AHG15J8AM0) .BETA.-D-GLUCOPYRANOSE (UNII: J4R00M814D) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43419-083-31 2 in 1 CARTON 07/15/2019 10/01/2022 1 15 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 07/15/2019 10/01/2022 SULWHASOO PERFECTING CUSHION NO. 11
octinoxate, titanium dioxide, and zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43419-074 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.1 g in 30 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.245 g in 30 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 2.94 g in 30 g Inactive Ingredients Ingredient Name Strength HYALURONATE SODIUM (UNII: YSE9PPT4TH) TOCOPHEROL (UNII: R0ZB2556P8) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) NIACINAMIDE (UNII: 25X51I8RD4) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) GLYCERIN (UNII: PDC6A3C0OX) PROPANEDIOL (UNII: 5965N8W85T) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRIMETHYLSILOXYSILICATE (M/Q 1.0-1.2) (UNII: 78GX033D7I) STEARIC ACID (UNII: 4ELV7Z65AP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ETHYLHEXYL PALMITATE (UNII: 2865993309) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) ISOSTEARIC ACID (UNII: X33R8U0062) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) POLYSORBATE 80 (UNII: 6OZP39ZG8H) CAPRYLYL GLYCOL (UNII: 00YIU5438U) FERRIC OXIDE RED (UNII: 1K09F3G675) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) FERROSOFERRIC OXIDE (UNII: XM0M87F357) EDETATE DISODIUM (UNII: 7FLD91C86K) ADENOSINE (UNII: K72T3FS567) HONEY (UNII: Y9H1V576FH) NELUMBO NUCIFERA FLOWER (UNII: 61W322NLDV) POLYGONATUM ODORATUM ROOT (UNII: KPF03O64AZ) LILIUM CANDIDUM BULB (UNII: AHG15J8AM0) .BETA.-D-GLUCOPYRANOSE (UNII: J4R00M814D) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43419-074-31 2 in 1 CARTON 07/15/2019 11/27/2021 1 15 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 07/15/2019 11/27/2021 SULWHASOO PERFECTING CUSHION NO. 25
octinoxate, titanium dioxide, and zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43419-079 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.1 g in 30 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.245 g in 30 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 2.94 g in 30 g Inactive Ingredients Ingredient Name Strength HYALURONATE SODIUM (UNII: YSE9PPT4TH) TOCOPHEROL (UNII: R0ZB2556P8) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) NIACINAMIDE (UNII: 25X51I8RD4) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) GLYCERIN (UNII: PDC6A3C0OX) PROPANEDIOL (UNII: 5965N8W85T) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRIMETHYLSILOXYSILICATE (M/Q 1.0-1.2) (UNII: 78GX033D7I) STEARIC ACID (UNII: 4ELV7Z65AP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ETHYLHEXYL PALMITATE (UNII: 2865993309) ISOSTEARIC ACID (UNII: X33R8U0062) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) POLYSORBATE 80 (UNII: 6OZP39ZG8H) CAPRYLYL GLYCOL (UNII: 00YIU5438U) FERRIC OXIDE RED (UNII: 1K09F3G675) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) FERROSOFERRIC OXIDE (UNII: XM0M87F357) EDETATE DISODIUM (UNII: 7FLD91C86K) ADENOSINE (UNII: K72T3FS567) HONEY (UNII: Y9H1V576FH) NELUMBO NUCIFERA FLOWER (UNII: 61W322NLDV) POLYGONATUM ODORATUM ROOT (UNII: KPF03O64AZ) LILIUM CANDIDUM BULB (UNII: AHG15J8AM0) .BETA.-D-GLUCOPYRANOSE (UNII: J4R00M814D) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43419-079-31 2 in 1 CARTON 07/15/2019 1 15 g in 1 CONTAINER; Type 0: Not a Combination Product 2 NDC:43419-079-39 2 in 1 CARTON 12/09/2019 12/02/2021 2 15 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 07/15/2019 Labeler - Amorepacific Corporation (631035289)