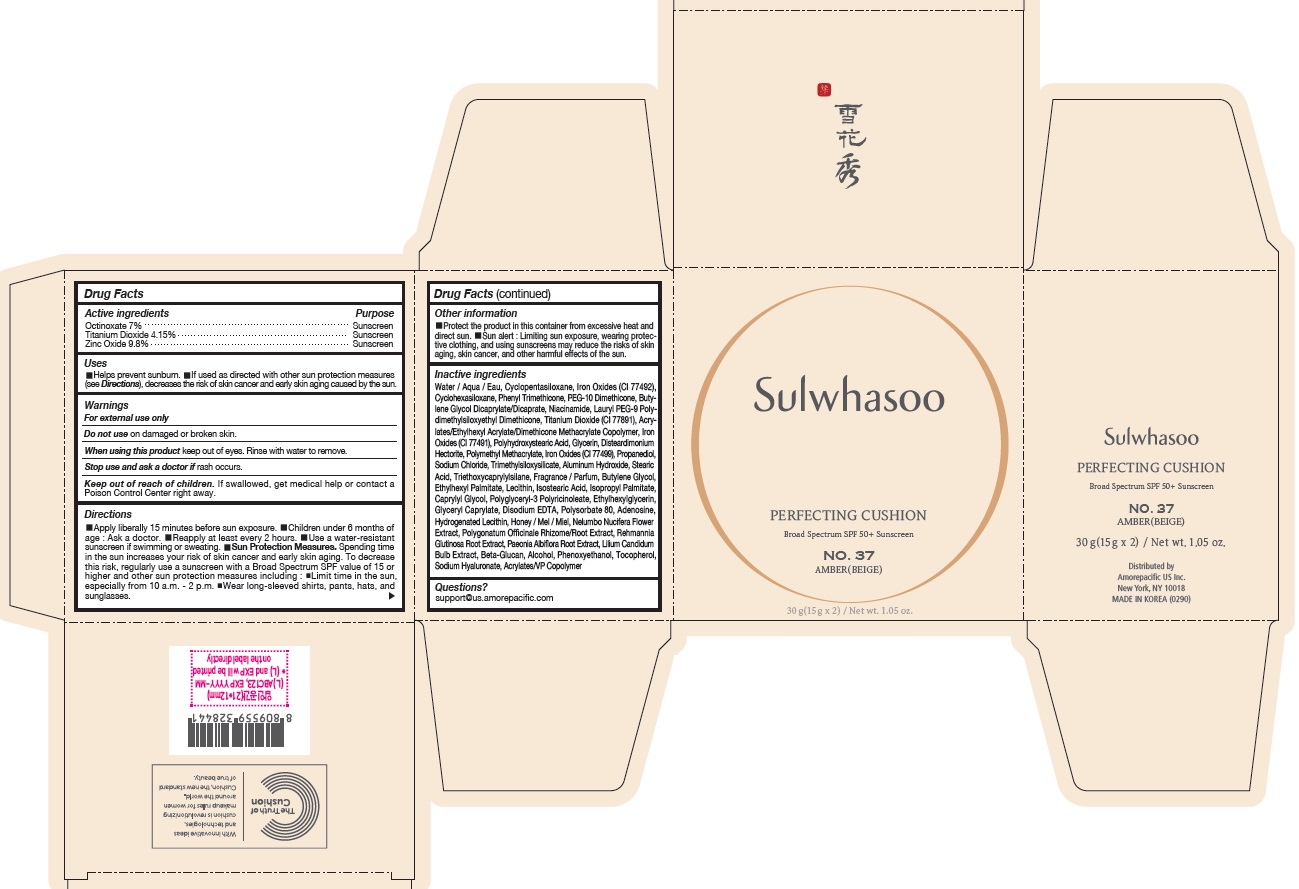
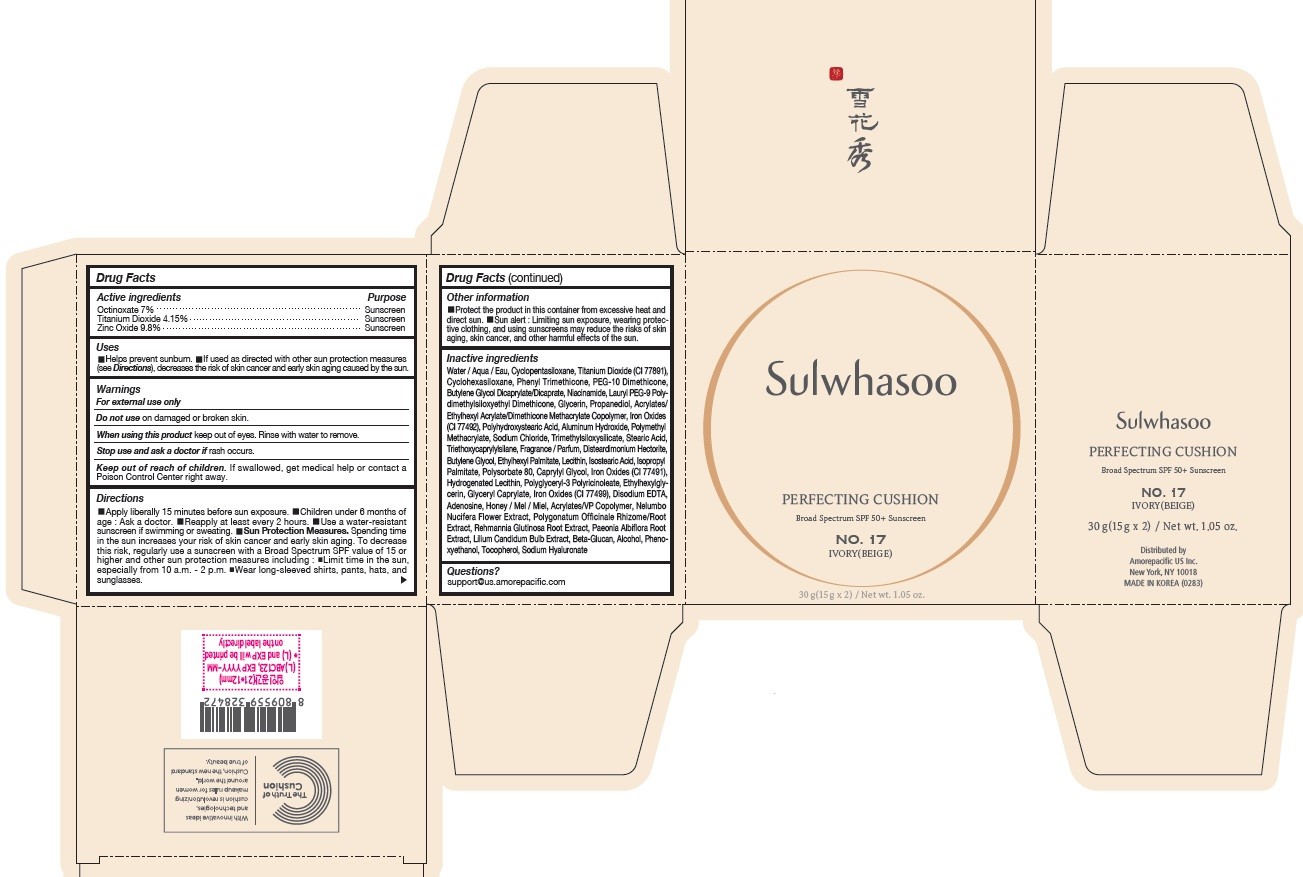
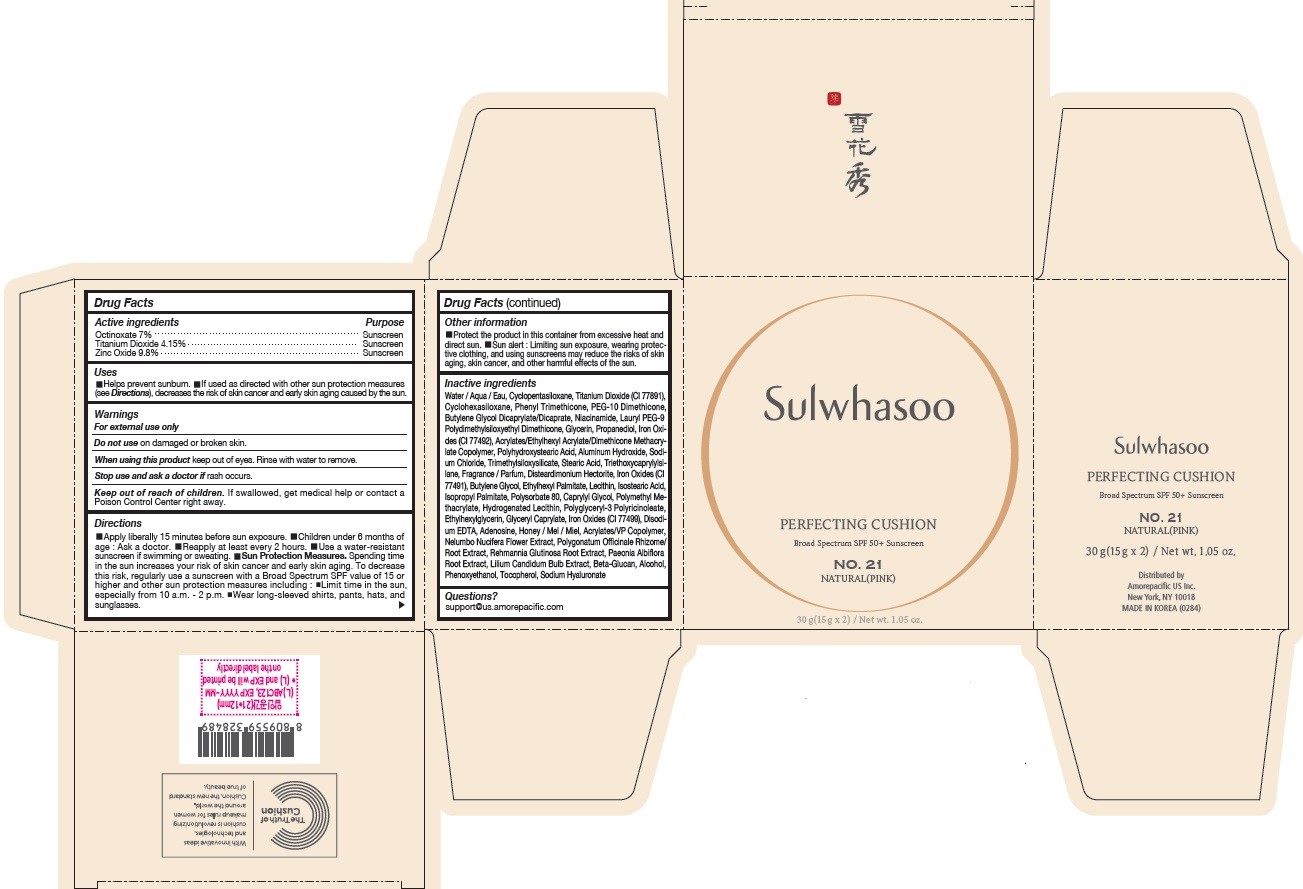
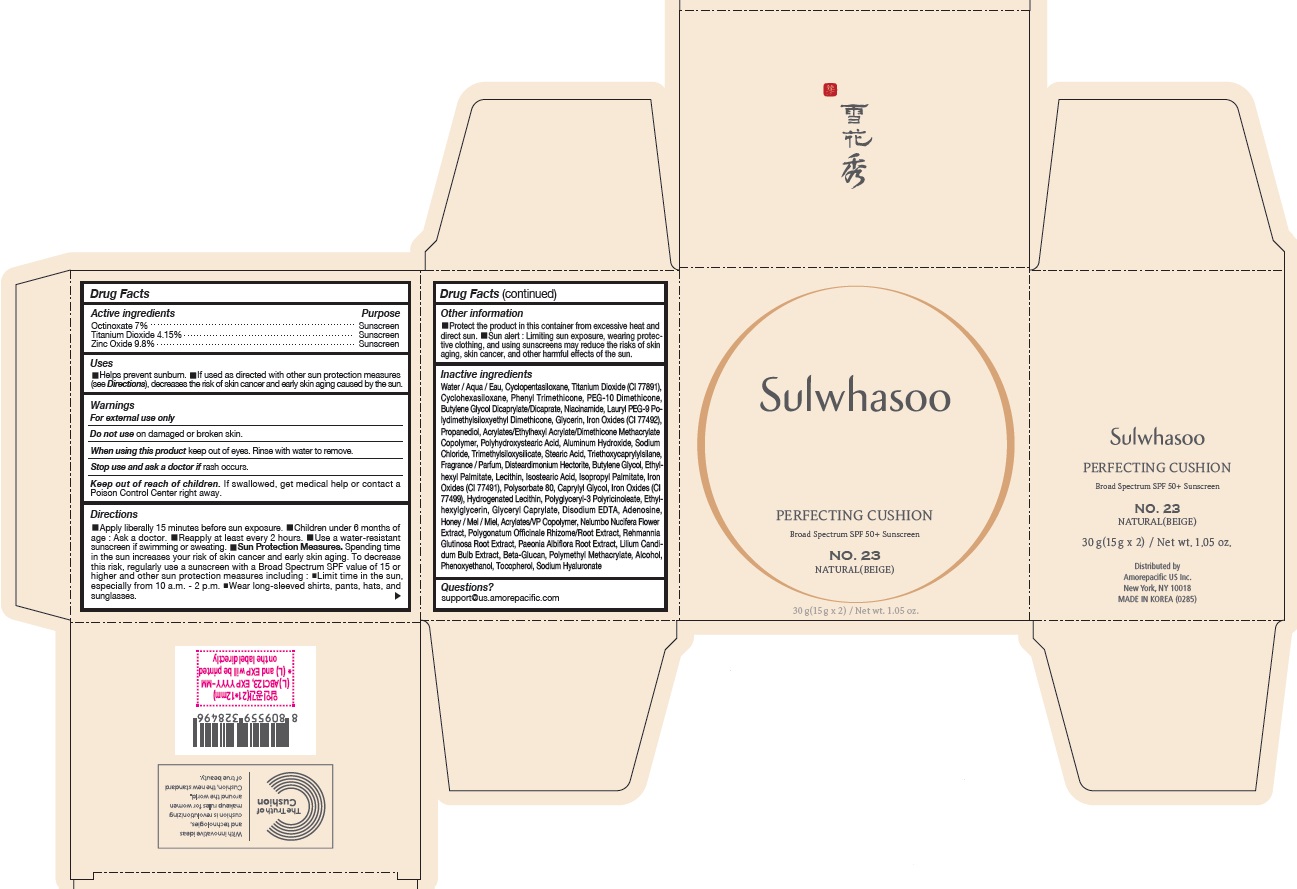
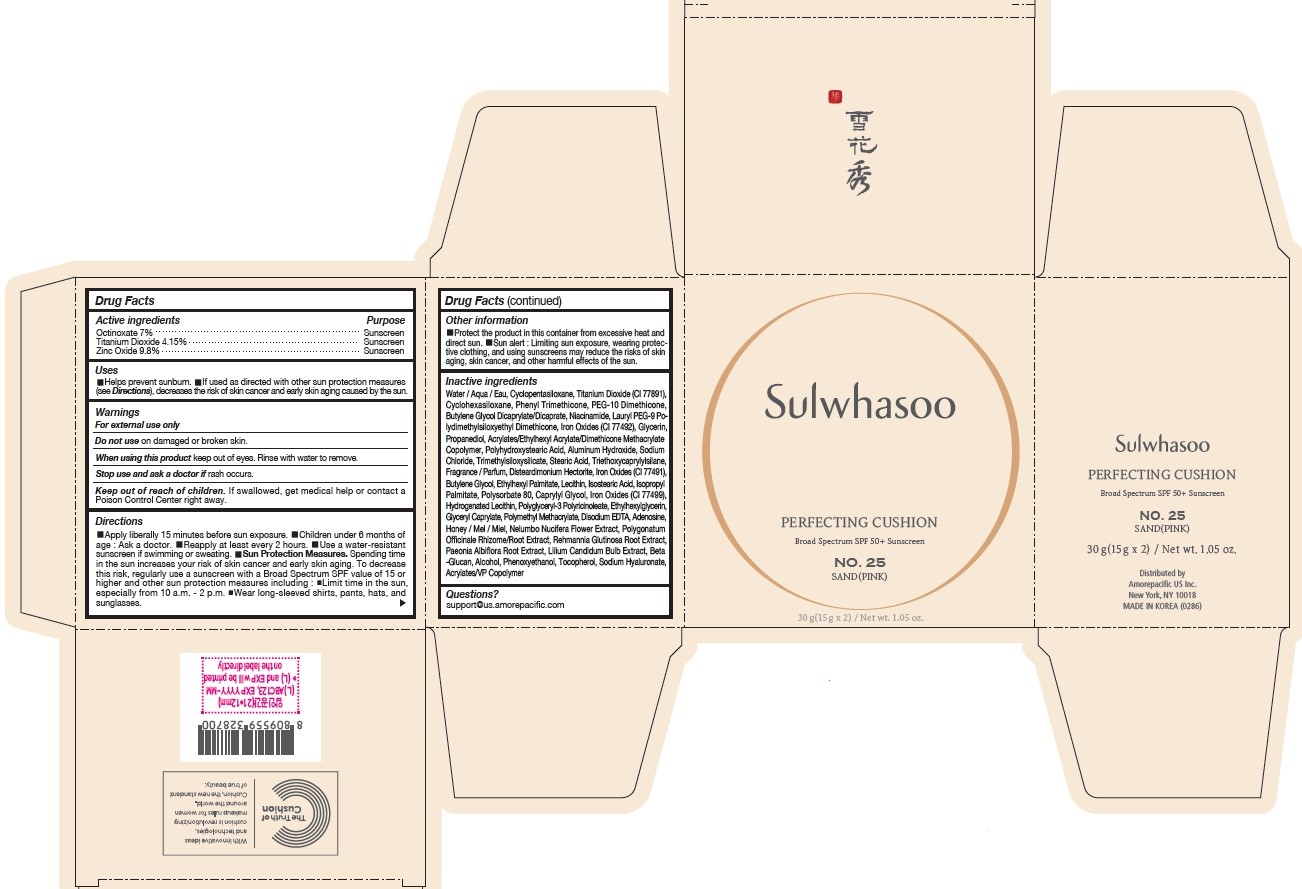
USES
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions ), decreases the risk of skin cancer and early skin aging caused by the sun
DIRECTIONS
- Apply liberally 15 minutes before sun exposure
- Children under 6 months of age : Ask a doctor.
- Reapply at least every 2 hours.
- Use a water-resistant sunscreen if swimming or sweating.
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including :
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
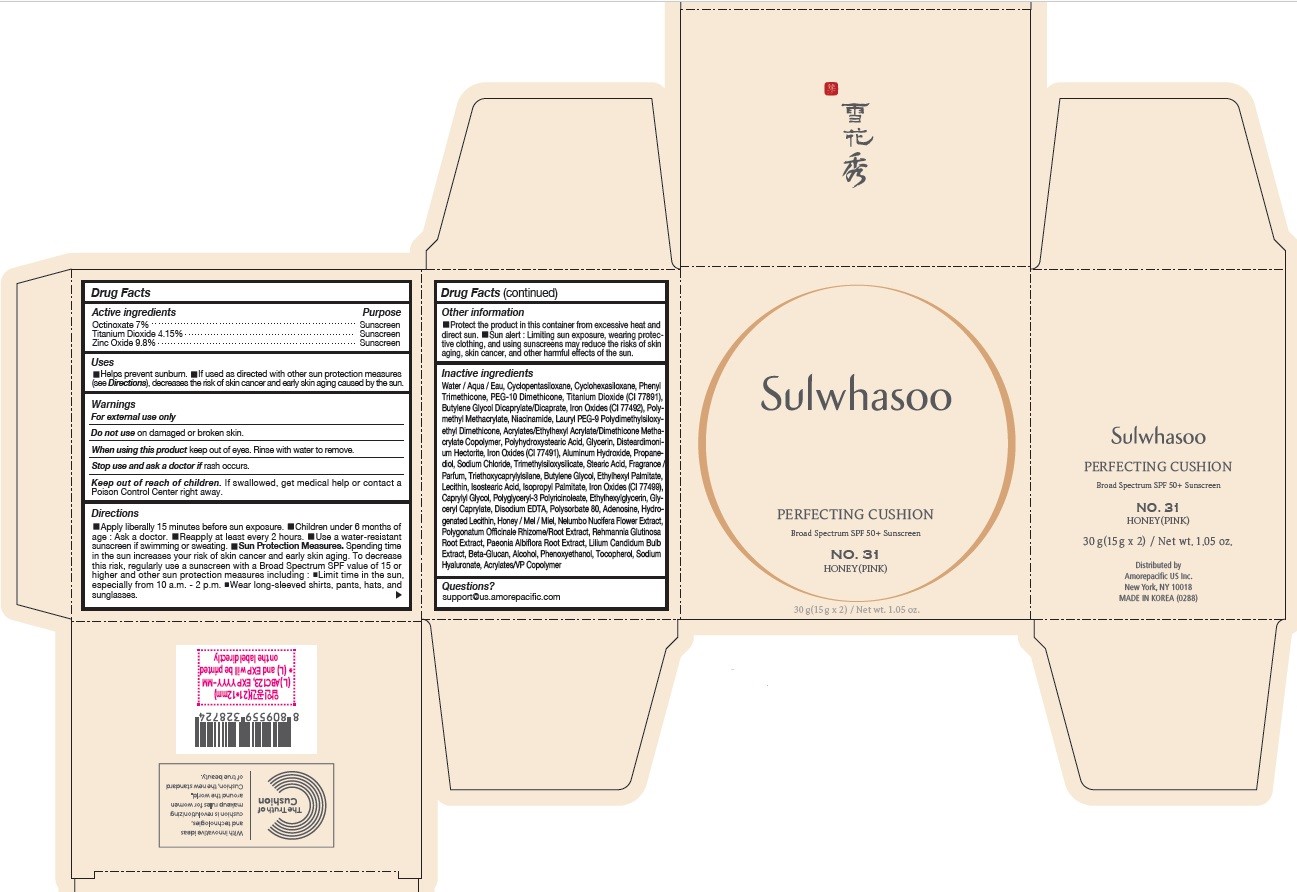
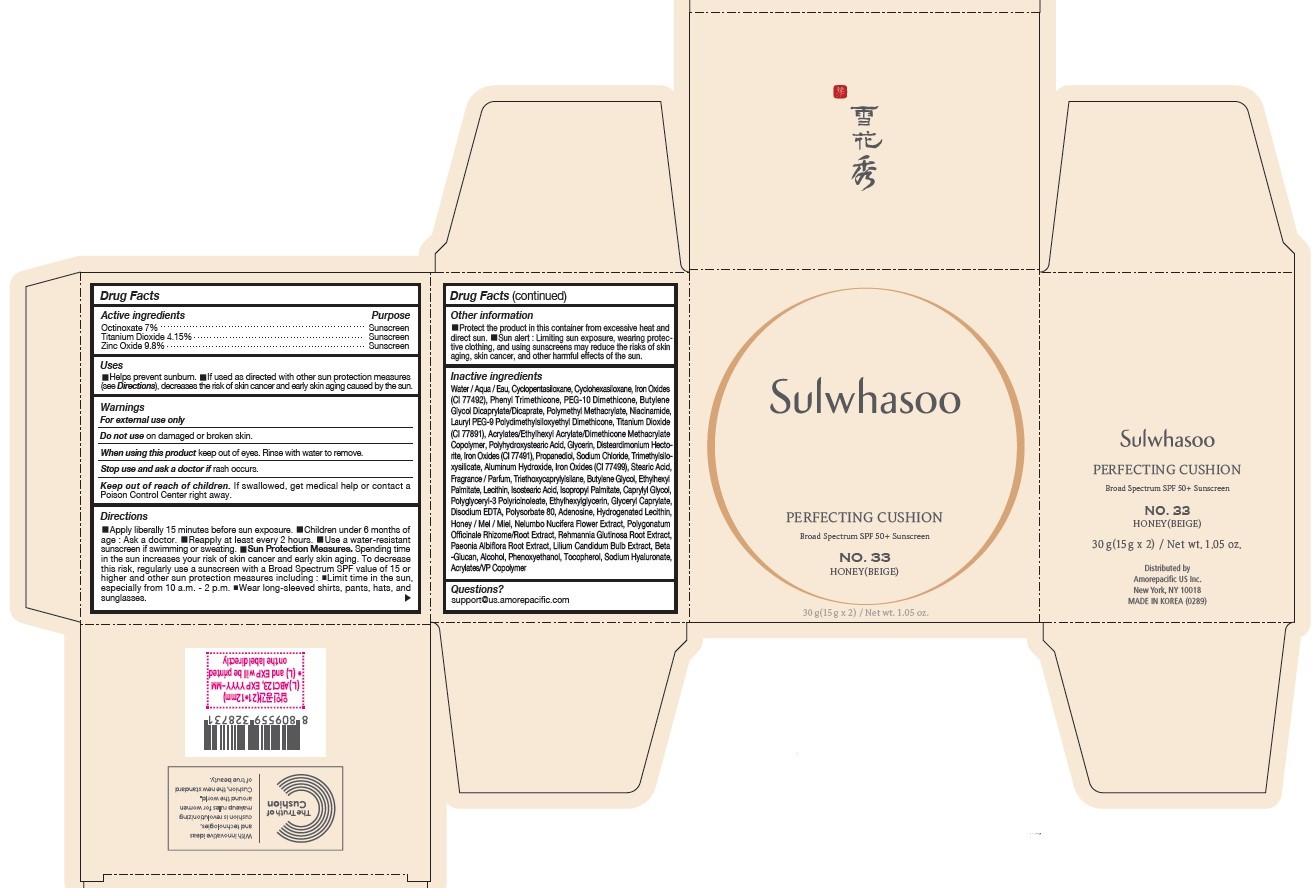
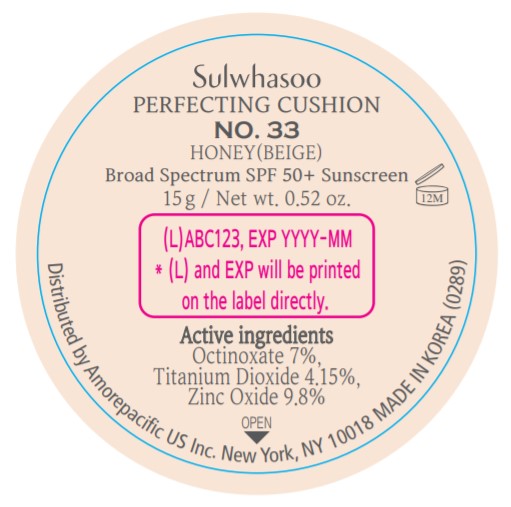
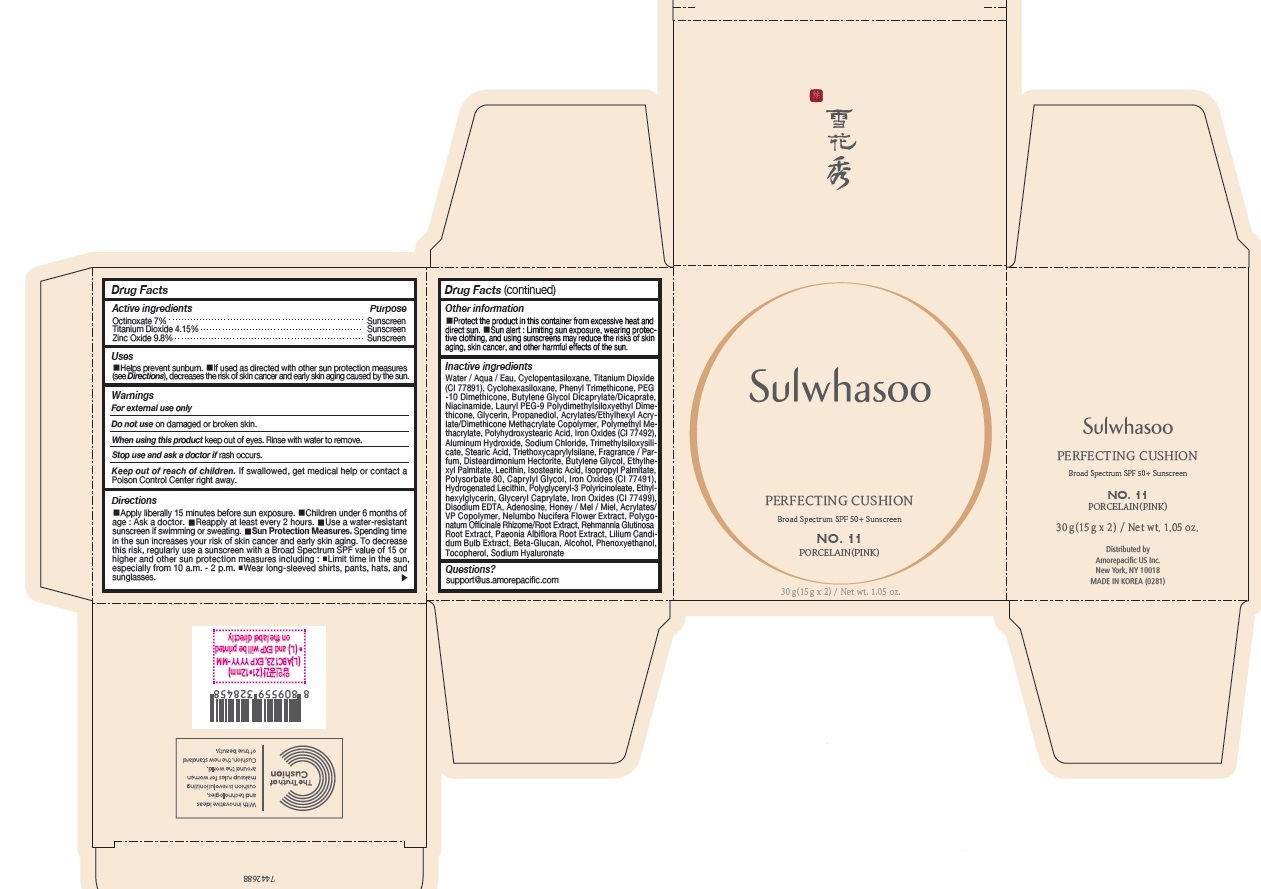
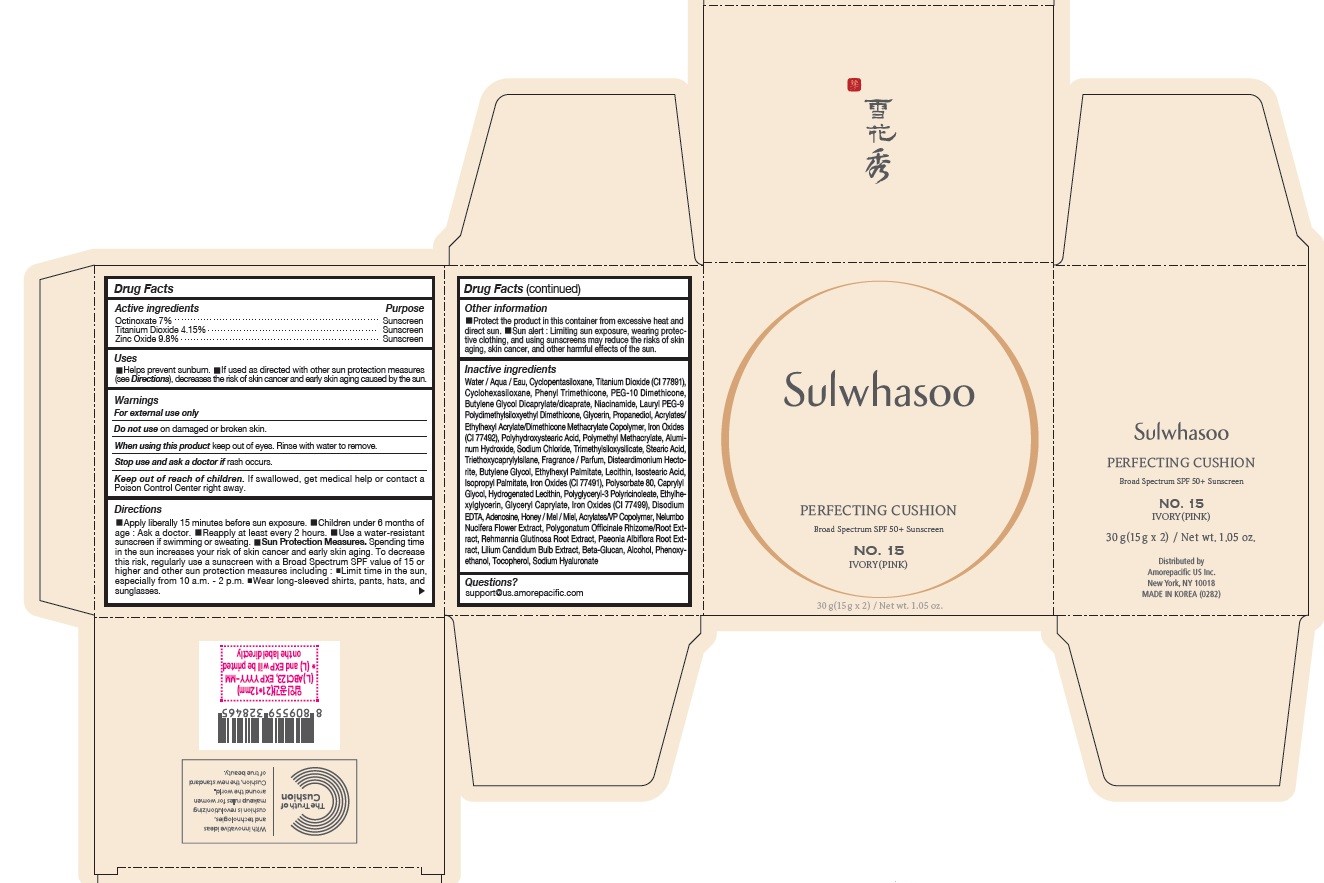
INACTIVE INGREDIENTS
Water / Aqua / Eau, Cyclopentasiloxane, Titanium Dioxide (CI 77891), Cyclohexasiloxane, Phenyl Trimethicone, PEG-10 Dimethicone, Butylene Glycol Dicaprylate/Dicaprate, Niacinamide, Lauryl PEG-9 Polydimethylsiloxyethyl Dimethicone, Glycerin, Propanediol, Acrylates/Ethylhexyl Acrylate/Dimethicone Methacrylate Copolymer, Polymethyl Methacrylate, Polyhydroxystearic Acid, Iron Oxides (CI 77492), Aluminum Hydroxide, Sodium Chloride, Trimethylsiloxysilicate, Stearic Acid, Triethoxycaprylylsilane, Fragrance / Parfum, Disteardimonium Hectorite, Butylene Glycol, Ethylhexyl Palmitate, Lecithin, Isostearic Acid, Isopropyl Palmitate, Polysorbate 80, Caprylyl Glycol, Iron Oxides (CI 77491), Hydrogenated Lecithin, Polyglyceryl-3 Polyricinoleate, Ethylhexylglycerin, Glyceryl Caprylate, Iron Oxides (CI 77499), Disodium EDTA, Adenosine, Honey / Mel / Miel, Acrylates/VP Copolymer, Nelumbo Nucifera Flower Extract, Polygonatum Officinale Rhizome/Root Extract, Rehmannia Glutinosa Root Extract, Paeonia Albiflora Root Extract, Lilium Candidum Bulb Extract, Beta-Glucan, Alcohol, Phenoxyethanol, Tocopherol, Sodium Hyaluronate
OTHER INFORMATION
- Protect the product in this container from excessive heat and direct sun.
- Sun alert : Limiting sun exposure, wearing protective clothing, and using sunscreens may reduce the risks of skin aging, skin cancer, and other harmful effects of the sun.
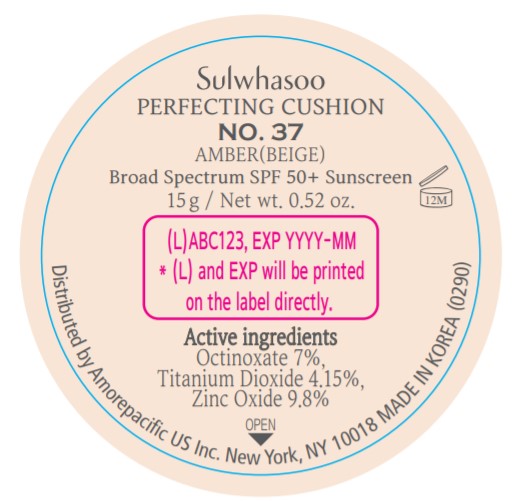
PRINCIPAL DISPLAY PANEL - NO. 11 PORCELAIN(PINK)
Sulwhasoo
PERFECTING CUSHION
BROAD SPECTRUM 50+ SUNSCREEN
NO. 11
PORCELAIN(PINK)
30 g(15 g x 2) / Net wt. 1.05 oz.


PRINCIPAL DISPLAY PANEL - NO. 15 IVORY(PINK)
Sulwhasoo
PERFECTING CUSHION
BROAD SPECTRUM 50+ SUNSCREEN
NO. 15
IVORY(PINK)
30 g(15 g x 2) / Net wt. 1.05 oz.


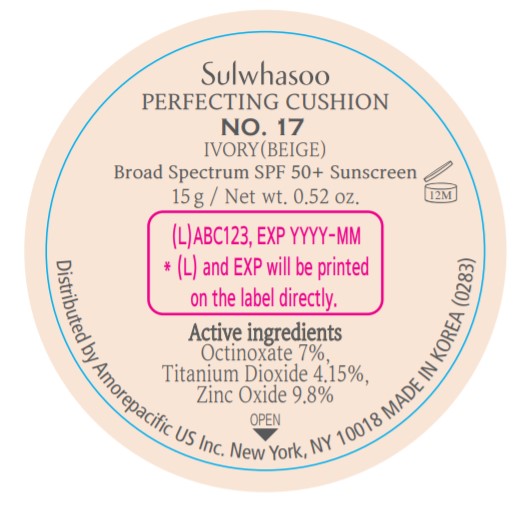
PRINCIPAL DISPLAY PANEL - NO. 17 IVORY(BEIGE)
Sulwhasoo
PERFECTING CUSHION
BROAD SPECTRUM SPF50+ SUNSCREEN
NO. 17
IVORY(BEIGE)
30 g(15 g x 2) / Net wt. 1.05 oz.

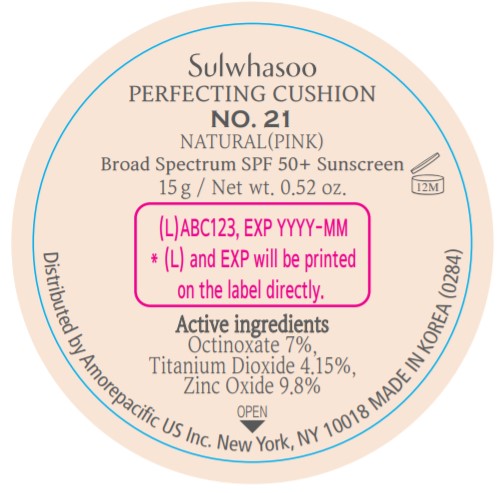
PRINCIPAL DISPLAY PANEL - NO. 21 NATURAL(PINK)
Sulwhasoo
PERFECTING CUSHION
BROAD SPECTRUM 50+ SUNSCREEN
NO. 21
NATURAL(PINK)
30 g(15 g x 2) / Net wt. 1.05 oz.

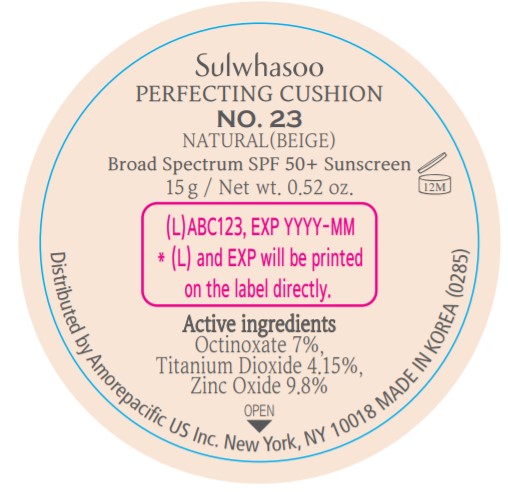
PRINCIPAL DISPLAY PANEL - NO. 23 NATURAL(BEIGE)
Sulwhasoo
PERFECTING CUSHION
BROAD SPECTRUM 50+ SUNSCREEN
NO. 23
NATURAL(BEIGE)
30 g(15 g x 2) / Net wt. 1.05 oz.

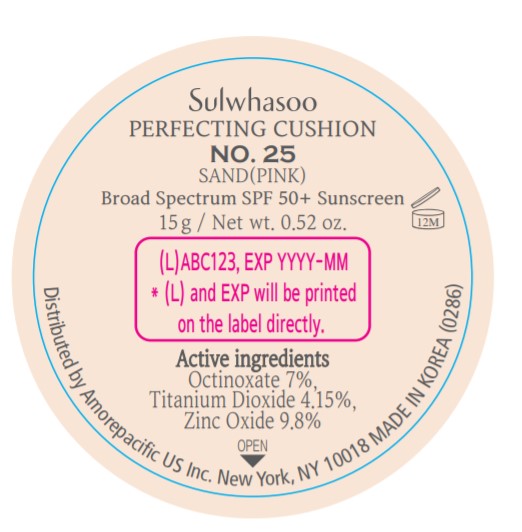
PRINCIPAL DISPLAY PANEL - NO. 25 SAND(PINK)
Sulwhasoo
PERFECTING CUSHION
BROAD SPECTRUM SPF50+ SUNSCREEN
NO. 25
SAND(PINK)
30 g(15 g x 2) / Net wt. 1.05 oz.

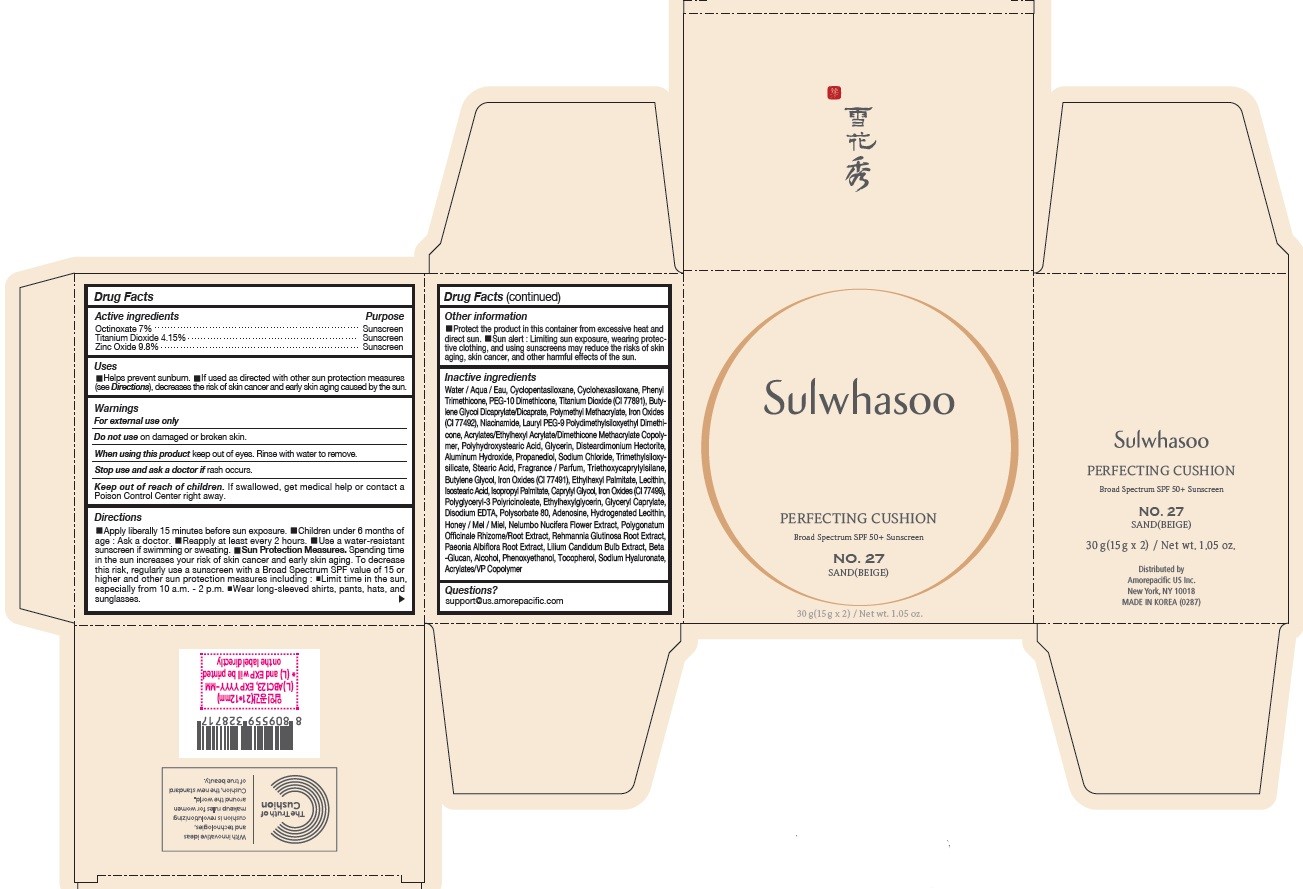
PRINCIPAL DISPLAY PANEL - NO. 27 SAND(BEIGE)
Sulwhasoo
PERFECTING CUSHION
BROAD SPECTRUM 50+ SUNSCREEN
NO. 27
SAND(BEIGE)
30 g(15 g x 2) / Net wt. 1.05 oz.


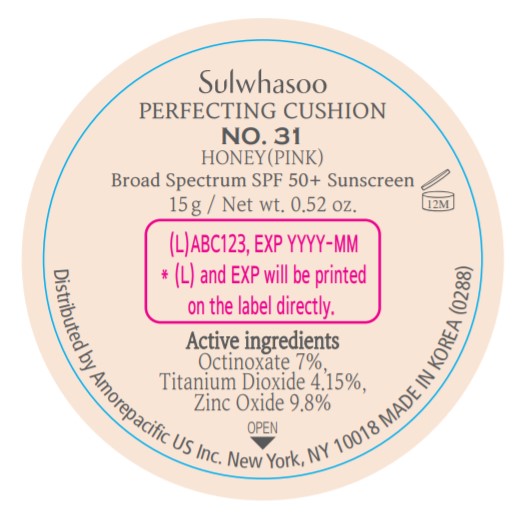
PRINCIPAL DISPLAY PANEL - NO. 31 HONEY(PINK)
Sulwhasoo
PERFECTING CUSHION
BROAD SPECTRUM 50+ SUNSCREEN
NO. 31
HONEY(PINK)
30 g(15 g x 2) / Net wt. 1.05 oz.