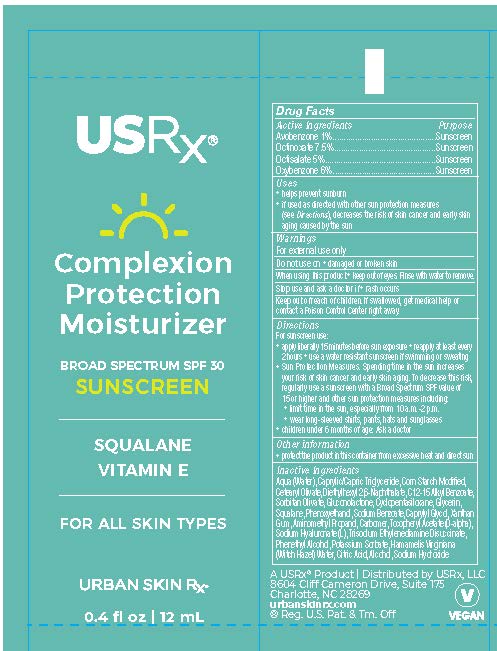
Label: COMPLEXION PROTECTION MOISTURIZER SPF- benzophenone, avobenzone, octinoxate, octisalate cream cream
- NDC Code(s): 70809-1902-1, 70809-1902-2
- Packager: USRX LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
PRINCIPAL DISPLAY PANEL

Aqua (Water), Caprylic/Capric Triglyceride, Corn Starch Modified, Cetearyl Olivate, Diethylhexyl 2,6-Naphthalate, C12-15 Alkyl Benzoate, Sorbitan Olivate, Gluconolactone, Squalane, Glycerin, Cyclopentasiloxane, Tocopheryl Acetate (D-alpha), Sodium Hyaluronate (L), Xanthan Gum, Carbomer, Aminomethyl Propanol, Sodium Benzoate, Phenethyl Alcohol, Caprylyl Glycol, Phenoxyethanol, Trisodium Ethylenediamine Disuccinate, Potassium Sorbate
-
INGREDIENTS AND APPEARANCE
COMPLEXION PROTECTION MOISTURIZER SPF
benzophenone, avobenzone, octinoxate, octisalate cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70809-1902 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 mg in 100 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 mg in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 1 mg in 100 mL BENZOPHENONE (UNII: 701M4TTV9O) (BENZOPHENONE - UNII:701M4TTV9O) BENZOPHENONE 3.6 mg in 100 mL Inactive Ingredients Ingredient Name Strength XANTHAN GUM (UNII: TTV12P4NEE) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) SODIUM BENZOATE (UNII: OJ245FE5EU) CAPRYLYL GLYCOL (UNII: 00YIU5438U) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) STARCH, CORN (UNII: O8232NY3SJ) WATER (UNII: 059QF0KO0R) CETEARYL OLIVATE (UNII: 58B69Q84JO) DIETHYLHEXYL 2,6-NAPHTHALATE (UNII: I0DQJ7YGXM) HYALURONATE SODIUM (UNII: YSE9PPT4TH) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) GLYCERIN (UNII: PDC6A3C0OX) SQUALANE (UNII: GW89575KF9) GLUCONOLACTONE (UNII: WQ29KQ9POT) SORBITAN OLIVATE (UNII: MDL271E3GR) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70809-1902-1 50 mL in 1 TUBE; Type 0: Not a Combination Product 07/08/2019 2 NDC:70809-1902-2 12 mL in 1 TUBE; Type 0: Not a Combination Product 02/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 07/08/2019 Labeler - USRX LLC (115270633) Registrant - USRX LLC (115270633)