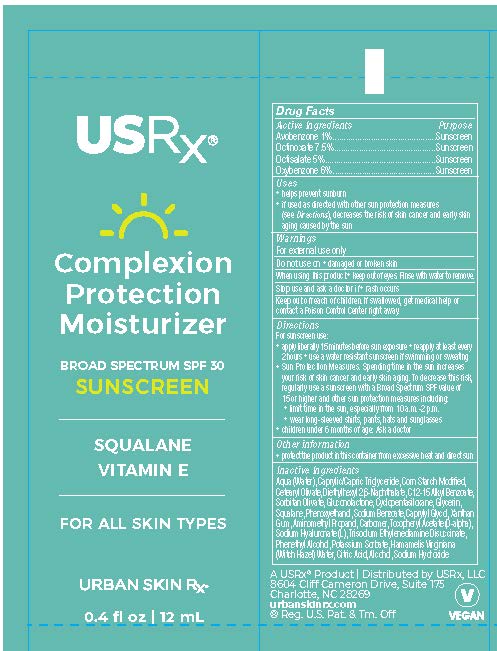

Aqua (Water), Caprylic/Capric Triglyceride, Corn Starch Modified, Cetearyl Olivate, Diethylhexyl 2,6-Naphthalate, C12-15 Alkyl Benzoate, Sorbitan Olivate, Gluconolactone, Squalane, Glycerin, Cyclopentasiloxane, Tocopheryl Acetate (D-alpha), Sodium Hyaluronate (L), Xanthan Gum, Carbomer, Aminomethyl Propanol, Sodium Benzoate, Phenethyl Alcohol, Caprylyl Glycol, Phenoxyethanol, Trisodium Ethylenediamine Disuccinate, Potassium Sorbate