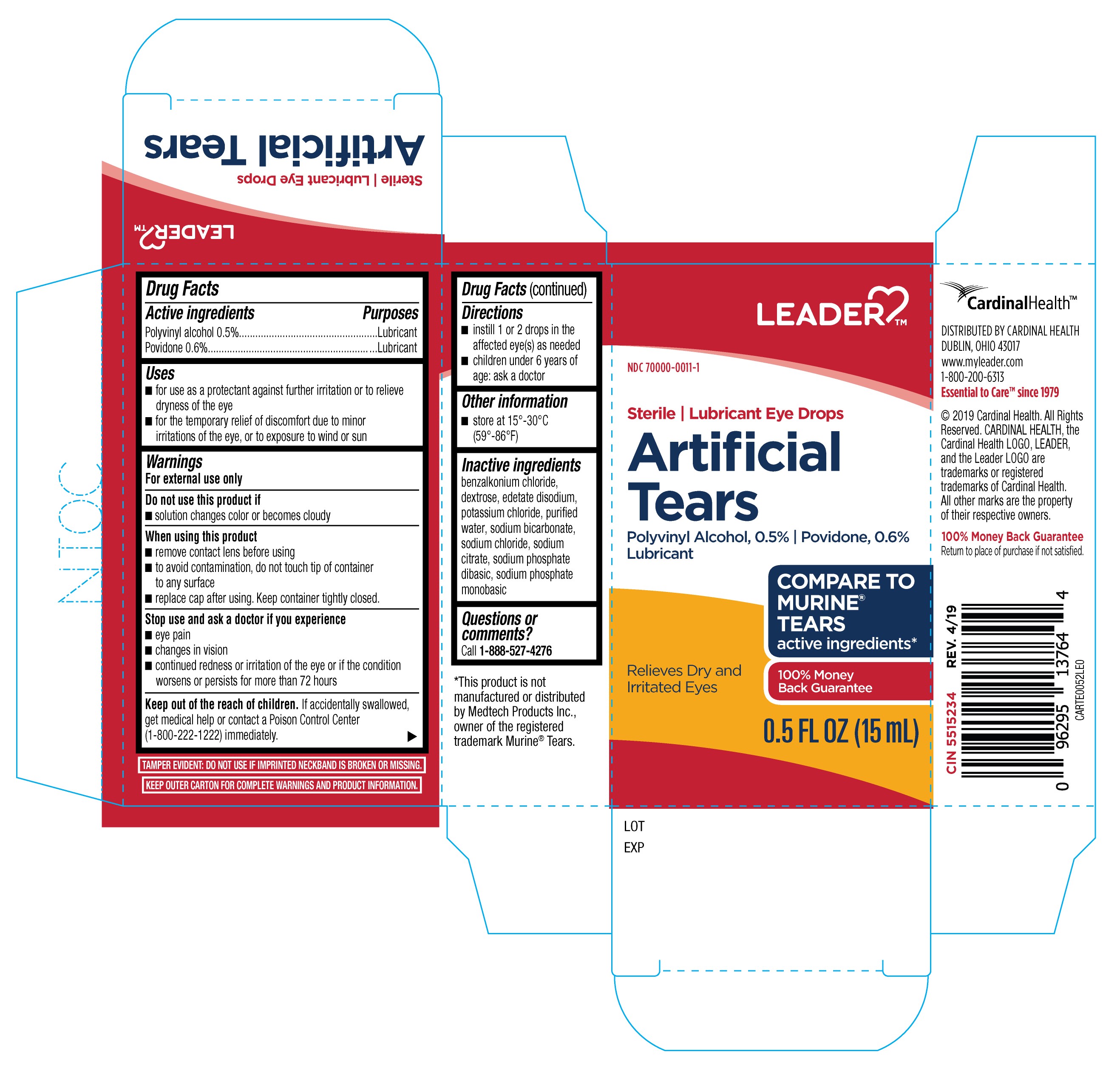
Label: LEADER ARTIFICIAL TEARS 15 ML- polyvinyl alcohol, povidone solution/ drops
- NDC Code(s): 70000-0011-1
- Packager: Cardinal Health
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purposes
- Uses
-
Warnings
For external use only
When using this product
- remove contact lens before using
- to avoid contamination, do not touch tip of container to any surface
- replace cap after using. Keep container tightly closed.
- Directions
- Other Information
- Inactive Ingredients
- Questions or comments?
- Leader Artificial Tears 15mL
-
INGREDIENTS AND APPEARANCE
LEADER ARTIFICIAL TEARS 15 ML
polyvinyl alcohol, povidone solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70000-0011 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) (POLYVINYL ALCOHOL, UNSPECIFIED - UNII:532B59J990) POLYVINYL ALCOHOL, UNSPECIFIED 0.5 g in 100 mL POVIDONE (UNII: FZ989GH94E) (POVIDONE - UNII:FZ989GH94E) POVIDONE 0.6 g in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM BICARBONATE (UNII: 8MDF5V39QO) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM PHOSPHATE, MONOBASIC, ANHYDROUS (UNII: KH7I04HPUU) DEXTROSE (UNII: IY9XDZ35W2) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM (UNII: 7FLD91C86K) WATER (UNII: 059QF0KO0R) SODIUM CITRATE (UNII: 1Q73Q2JULR) POTASSIUM CHLORIDE (UNII: 660YQ98I10) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70000-0011-1 1 in 1 BOX 07/03/2019 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 07/03/2019 Labeler - Cardinal Health (063997360) Registrant - KC Pharmaceuticals, Inc. (174450460) Establishment Name Address ID/FEI Business Operations KC Pharmaceuticals, Inc. 174450460 manufacture(70000-0011) , pack(70000-0011) , label(70000-0011)