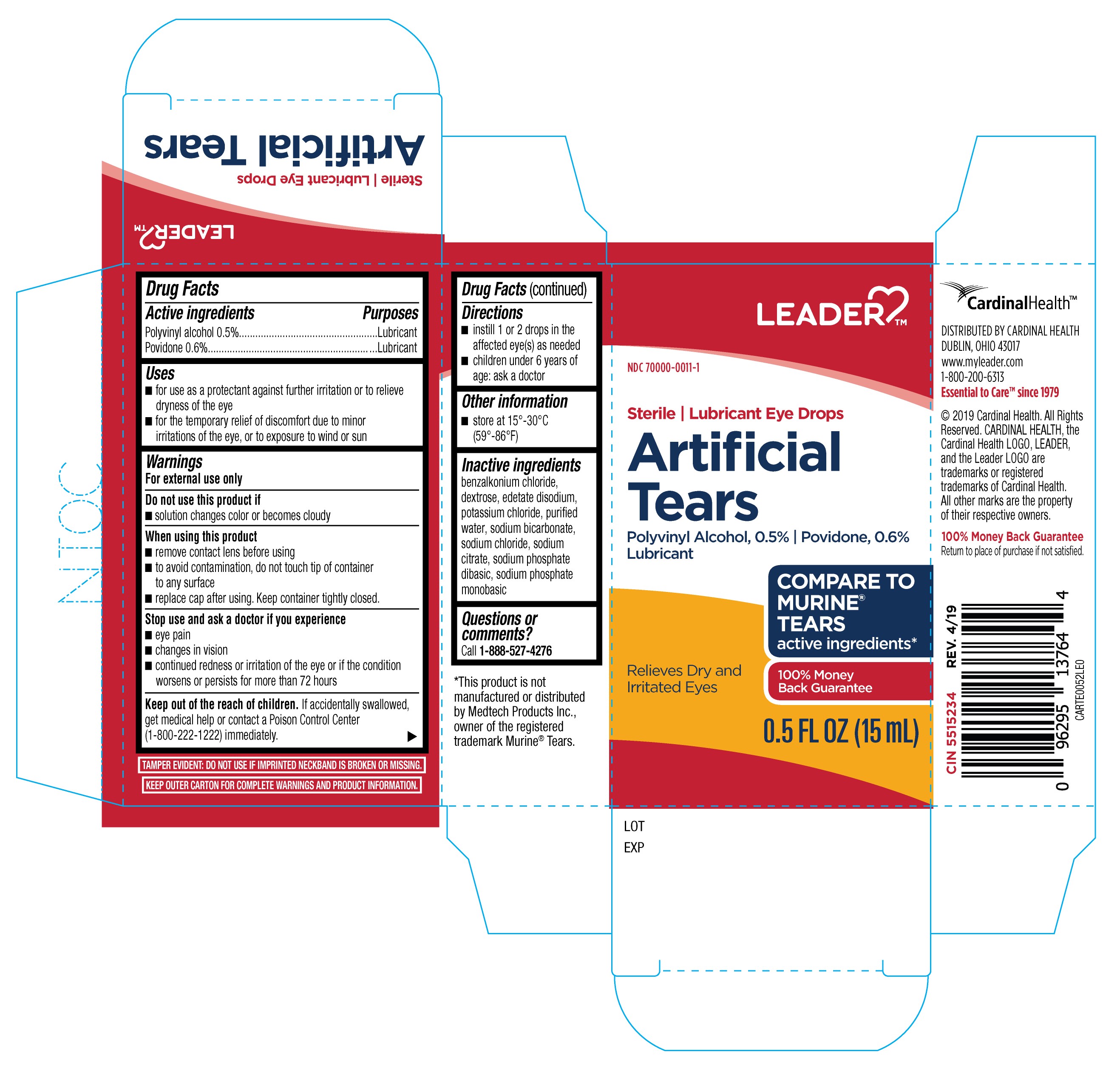
Active Ingredients
Polyvinyl alcohol 0.5%
Povidone 0.6%
Purposes
Lubricant
Lubricant
Uses
- for use as a protectant against further irritation or to relieve dryness of the eye
- for the temporary relief of discomfort due to minor irritations of the eye, or to exposure to wind or sun
Warnings
For external use only
Do not use this product if
- solution changes color or becomes cloudy
When using this product
- remove contact lens before using
- to avoid contamination, do not touch tip of container to any surface
- replace cap after using. Keep container tightly closed.
Stop use and ask a doctor if you experience
- eye pain
- changes in vision
- continued redness or irritation of the eye or if the condition worsens or persists for more than 72 hours
Keep out of the reach of children.
if accidentally swallowed, get medical help or contact Poison Control Center (1-800-222-1222) immediately.
Directions
- instill 1 or 2 drops in the affected eye(s) as needed
- children under 6 years of age: ask a doctor
Other Information
- store at 15°-30°C (59°-86°F)
Inactive Ingredients
benzalkonium chloride, dextrose, edetate disodium, potassium chloride, purified water, sodium bicarbonate, sodium chloride, sodium citrate, sodium phosphate dibasic, sodium phosphate monobasic
Questions or comments?
Call 1-888-527-4276
Leader Artificial Tears 15mL