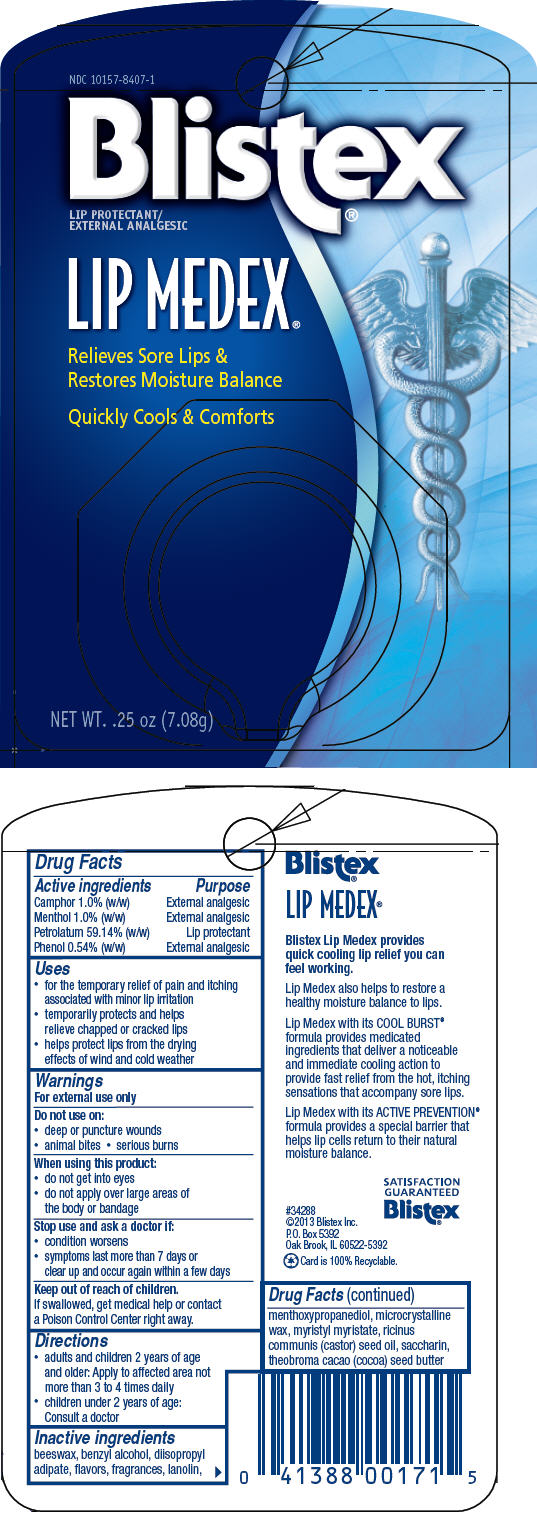
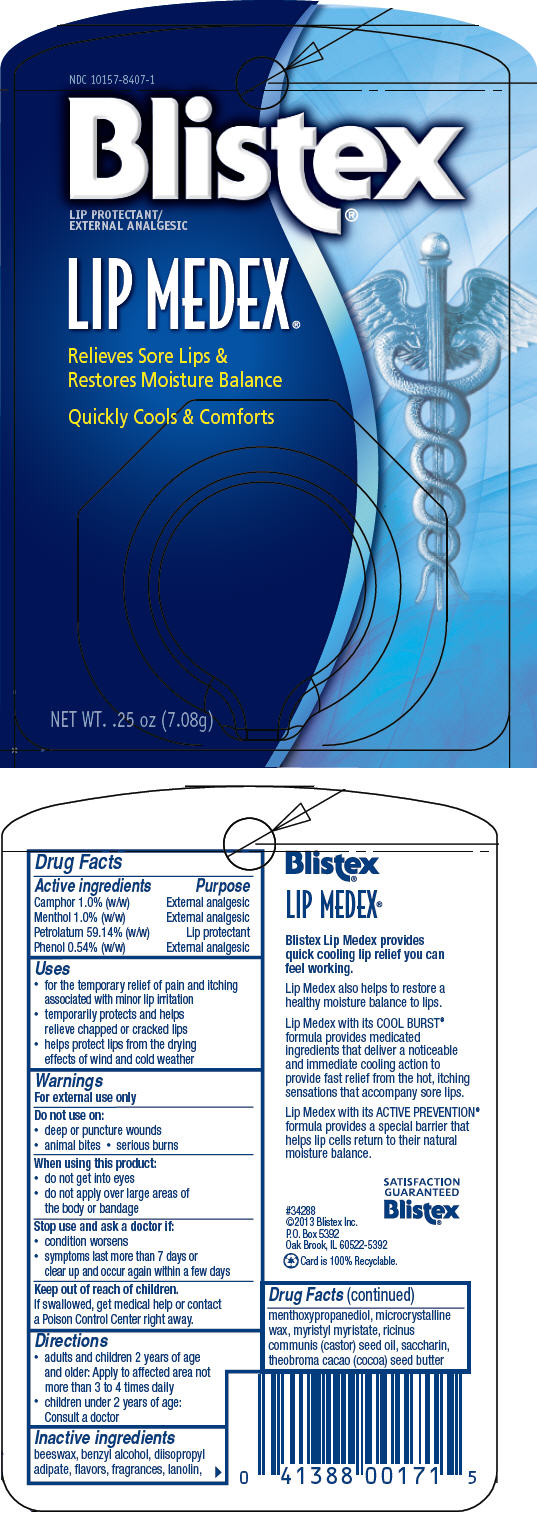
Label: BLISTEX LIP MEDEX (petrolatum, menthol, unspecified form, camphor- synthetic, and phenol paste
-
NDC Code(s):
10157-8407-1,
10157-8407-2,
10157-8407-3,
10157-8407-4, view more10157-8407-5
- Packager: Blistex Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL - 7.08 g Jar Blister Pack
-
INGREDIENTS AND APPEARANCE
BLISTEX LIP MEDEX
petrolatum, menthol, unspecified form, camphor (synthetic), and phenol pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10157-8407 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 59.14 g in 100 g MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 1 g in 100 g CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 1 g in 100 g PHENOL (UNII: 339NCG44TV) (PHENOL - UNII:339NCG44TV) PHENOL 0.6 g in 100 g Inactive Ingredients Ingredient Name Strength LANOLIN (UNII: 7EV65EAW6H) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) COCOA BUTTER (UNII: 512OYT1CRR) YELLOW WAX (UNII: 2ZA36H0S2V) CASTOR OIL (UNII: D5340Y2I9G) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) MENTHYL LACTATE, (-)- (UNII: 2BF9E65L7I) BENZYL ALCOHOL (UNII: LKG8494WBH) 3-((L-MENTHYL)OXY)PROPANE-1,2-DIOL (UNII: KD6TZ2QICH) MENTHYL SALICYLATE, (+/-)- (UNII: 43XOA705ZD) SACCHARIN (UNII: FST467XS7D) EUCALYPTUS OIL (UNII: 2R04ONI662) CLOVE OIL (UNII: 578389D6D0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10157-8407-1 1 in 1 BLISTER PACK 03/28/2017 1 7.08 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:10157-8407-2 1 in 1 BLISTER PACK 03/28/2017 2 10.75 g in 1 JAR; Type 0: Not a Combination Product 3 NDC:10157-8407-3 3 in 1 BLISTER PACK 11/29/2022 3 7.08 g in 1 JAR; Type 0: Not a Combination Product 4 NDC:10157-8407-4 7.08 g in 1 JAR; Type 0: Not a Combination Product 01/24/2024 5 NDC:10157-8407-5 10.75 g in 1 JAR; Type 0: Not a Combination Product 01/24/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M017 03/28/2017 Labeler - Blistex Inc. (005126354) Establishment Name Address ID/FEI Business Operations Blistex Inc. 005126354 MANUFACTURE(10157-8407)