BLISTEX LIP MEDEX- petrolatum, menthol, unspecified form, camphor (synthetic), and phenol paste
Blistex Inc.
----------
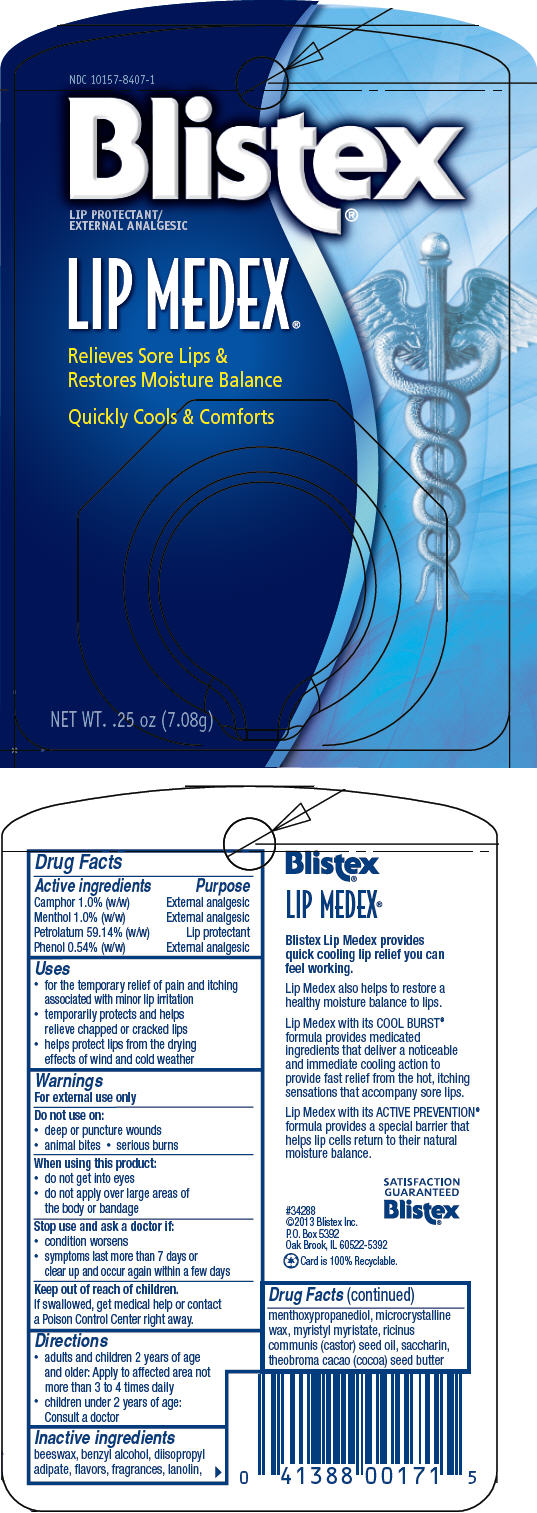
| Active ingredients | Purpose |
| Camphor 1.0% (w/w) | External analgesic |
| Menthol 1.0% (w/w) | External analgesic |
| Petrolatum 59.14% (w/w) | Lip protectant |
| Phenol 0.54% (w/w) | External analgesic |
Uses
- for the temporary relief of pain and itching associated with minor lip irritation
- temporarily protects and helps relieve chapped or cracked lips
- helps protect lips from the drying effects of wind and cold weather
Warnings
For external use only
Do not use on
- deep or puncture wounds
- animal bites
- serious burns
When using this product
- do not get into eyes
- do not apply over large areas of the body or bandage
Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 2 years of age and older: Apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: Consult a doctor
Inactive ingredients
beeswax, benzyl alcohol, diisopropyl adipate, flavors, fragrances, lanolin, menthoxypropanediol, microcrystalline wax, myristyl myristate, ricinus communis (castor) seed oil, saccharin, theobroma cacao (cocoa) seed butter
PRINCIPAL DISPLAY PANEL - 7.08 g Jar Blister Pack
NDC 10157-8407-1
Blistex®
LIP PROTECTANT/
EXTERNAL ANALGESIC
LIP MEDEX®
Relieves Sore Lips &
Restores Moisture Balance
Quickly Cools & Comforts
NET WT. .25 oz (7.08g)
Blistex Inc.