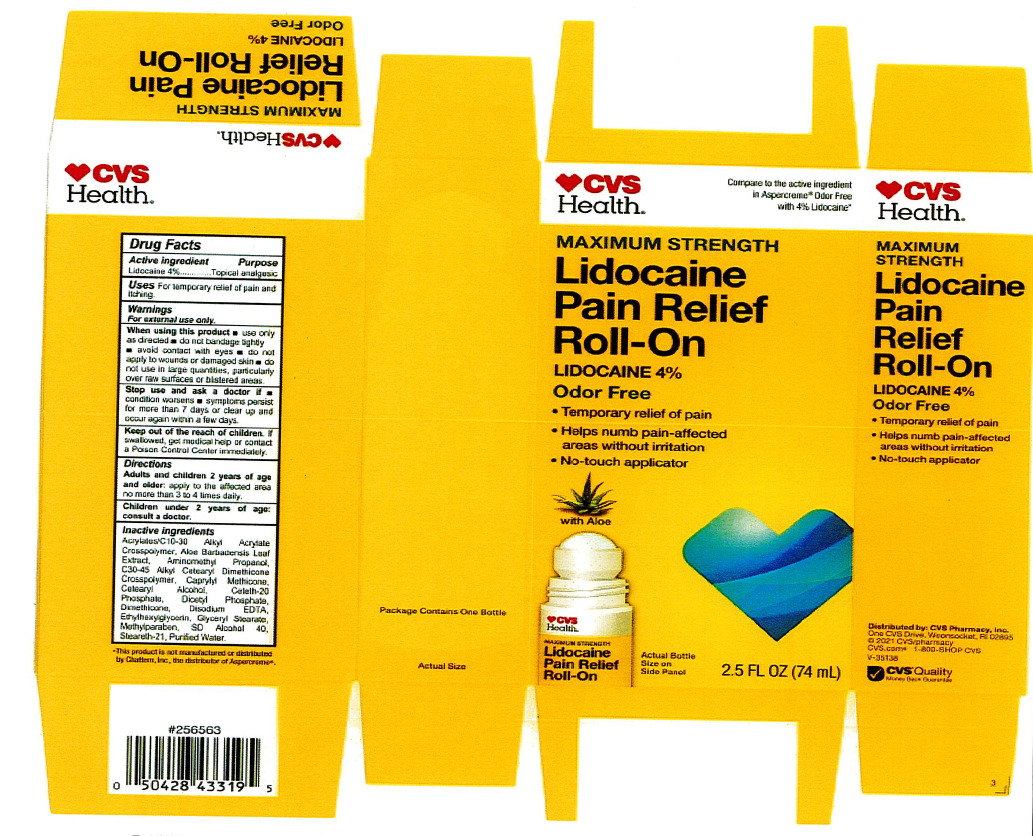
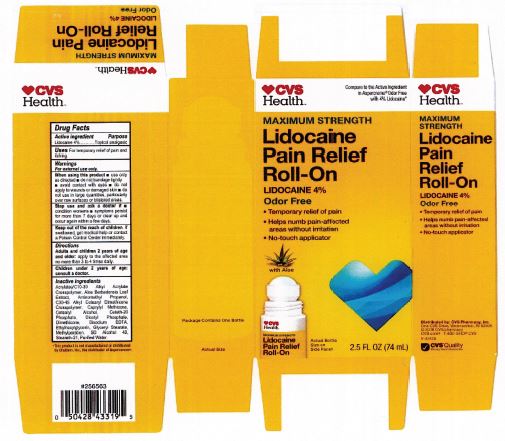
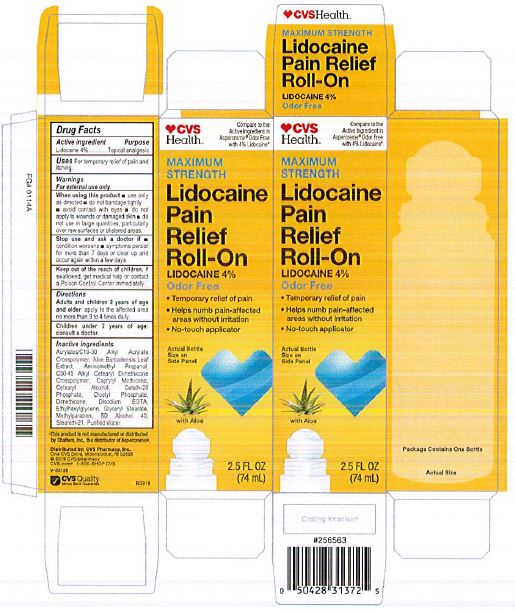
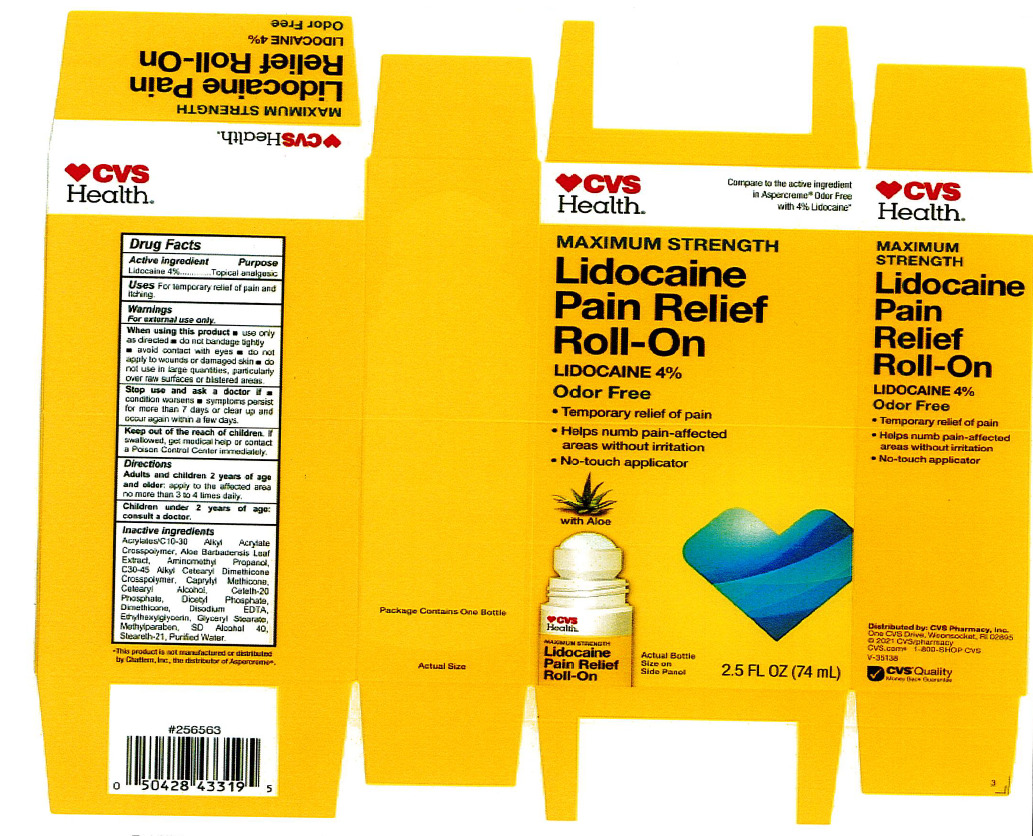
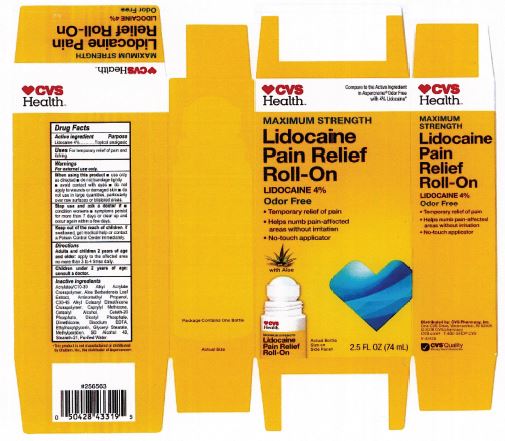
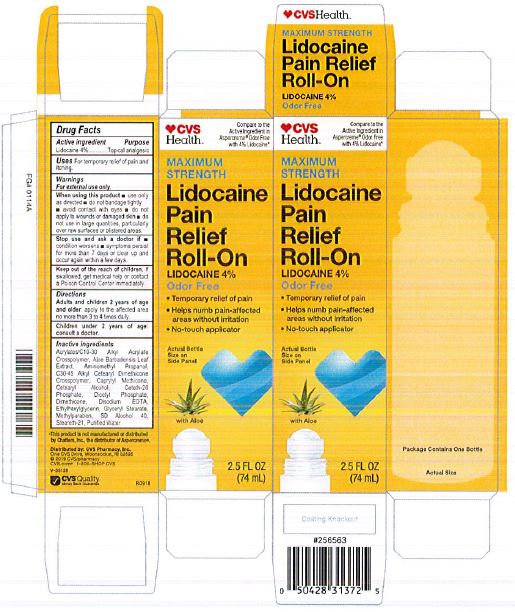
Label: LIDOCAINE PAIN RELIEF ROLL-ON- lidocaine 4% liquid
- NDC Code(s): 69842-029-97
- Packager: CVS Pharmacy
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
acrylates/C10-30 alkyl acrylate crosspolymer, aloe barbadensis leaf juice, aminomethyl propanol, C30-45 alkyl cetearyl dimethicone crosspolymer, caprylyl methicone, cetearyl alcohol, ceteth-20 phosphate, dicetyl phosphate, dimethicone, disodium EDTA, ethylhexylglycerin, glyceryl stearate, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, methylparaben, polysorbate 60, SD alcohol 40 (15%), steareth-2, steareth-21, water
- ACTIVE INGREDIENT
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LIDOCAINE PAIN RELIEF ROLL-ON
lidocaine 4% liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-029 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 4 g in 100 mL Inactive Ingredients Ingredient Name Strength AMINOMETHYLPROPANOL (UNII: LU49E6626Q) WATER (UNII: 059QF0KO0R) STEARETH-21 (UNII: 53J3F32P58) ALCOHOL (UNII: 3K9958V90M) METHYLPARABEN (UNII: A2I8C7HI9T) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) CARBOMER INTERPOLYMER TYPE A (55000 CPS) (UNII: 59TL3WG5CO) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) DIHEXADECYL PHOSPHATE (UNII: 2V6E5WN99N) CETETH-20 PHOSPHATE (UNII: 921FTA1500) DIMETHICONE (UNII: 92RU3N3Y1O) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-029-97 1 in 1 CARTON 06/05/2019 1 74 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 06/05/2019 Labeler - CVS Pharmacy (062312574) Registrant - Pharma Nobis, LLC (118564114) Establishment Name Address ID/FEI Business Operations Pharma Nobis, LLC 118564114 manufacture(69842-029) , analysis(69842-029) , label(69842-029) , pack(69842-029)