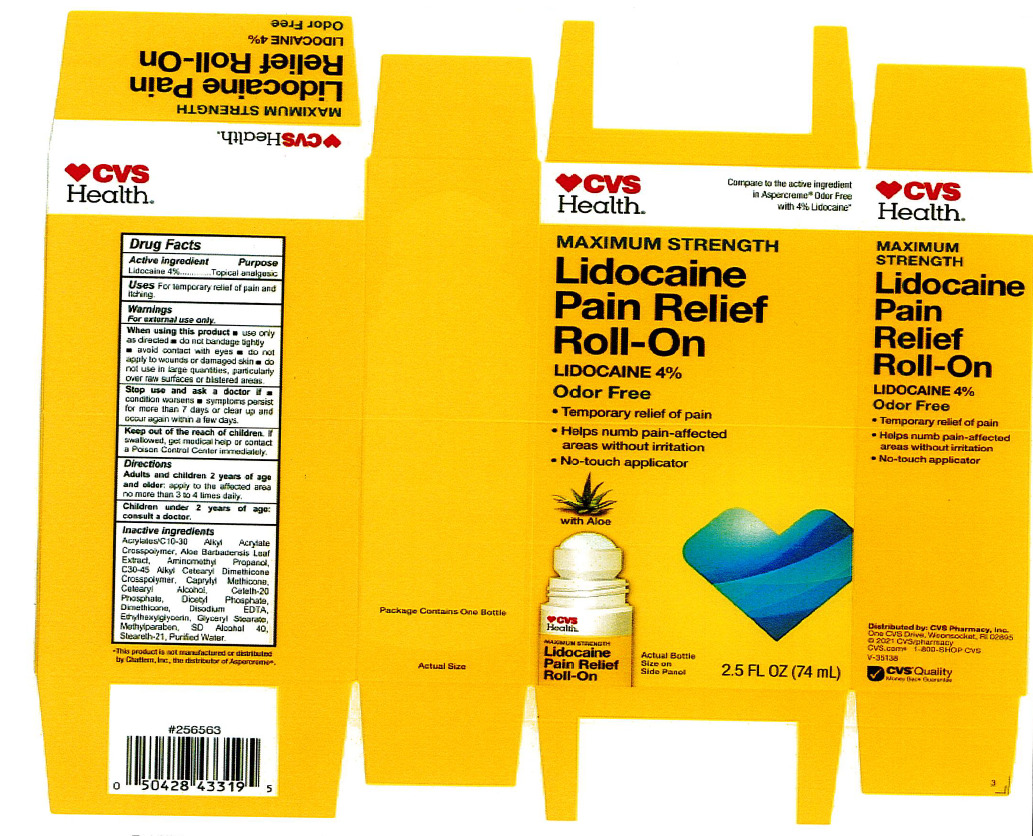
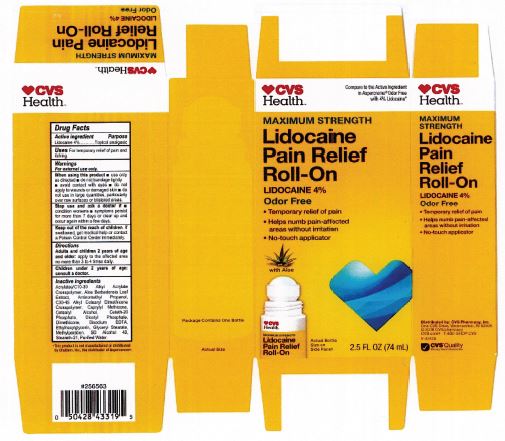
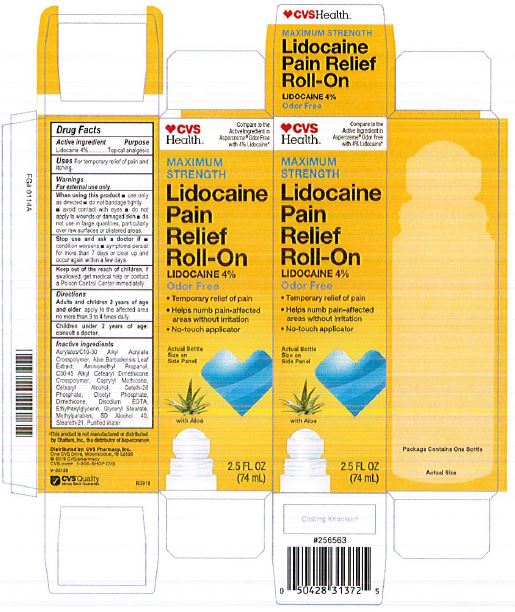
Adults and children 2 years of age and older: apply to the affected area no more than 3 to 4 times daily.
Children under 2 years of age: consult a doctor.
acrylates/C10-30 alkyl acrylate crosspolymer, aloe barbadensis leaf juice, aminomethyl propanol, C30-45 alkyl cetearyl dimethicone crosspolymer, caprylyl methicone, cetearyl alcohol, ceteth-20 phosphate, dicetyl phosphate, dimethicone, disodium EDTA, ethylhexylglycerin, glyceryl stearate, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, methylparaben, polysorbate 60, SD alcohol 40 (15%), steareth-2, steareth-21, water
For External Use Only.
When using this product:
use only as directed
do not bandage tightly or use with a heating pad
avoid contact with eyes
do not apply to wounds or damaged skin
do not use in large quantities
partifcularly over raw surfaces or blistered areas