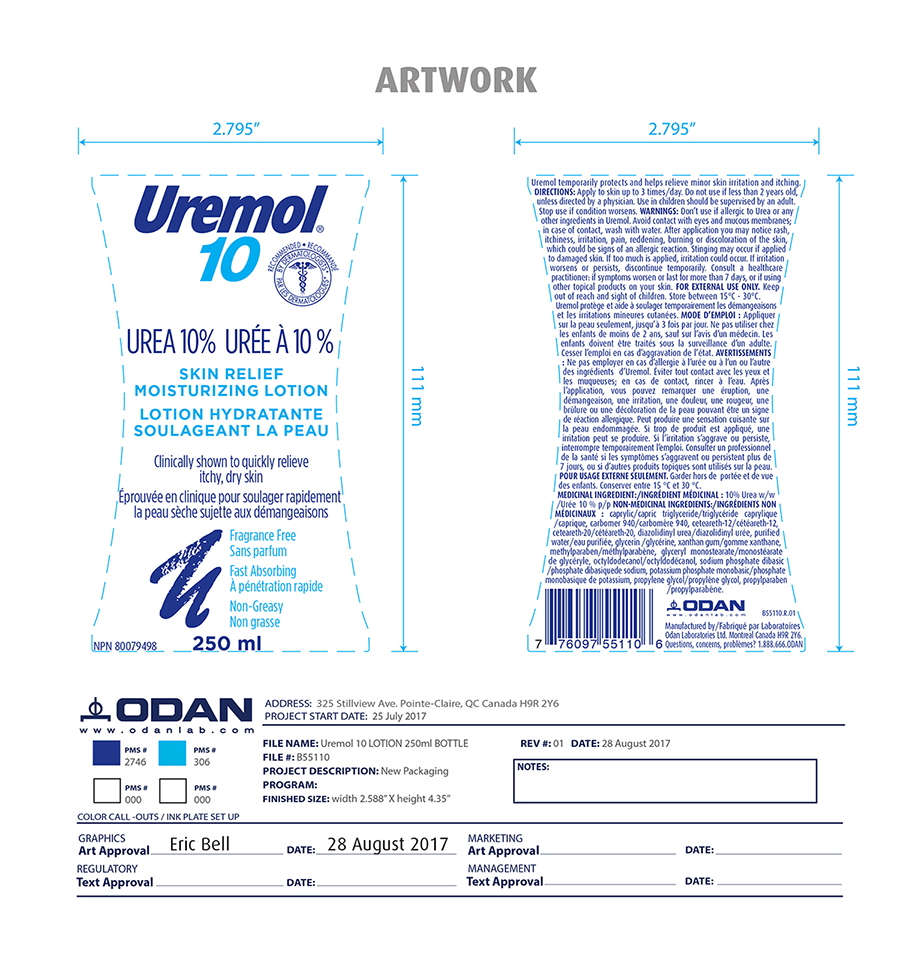
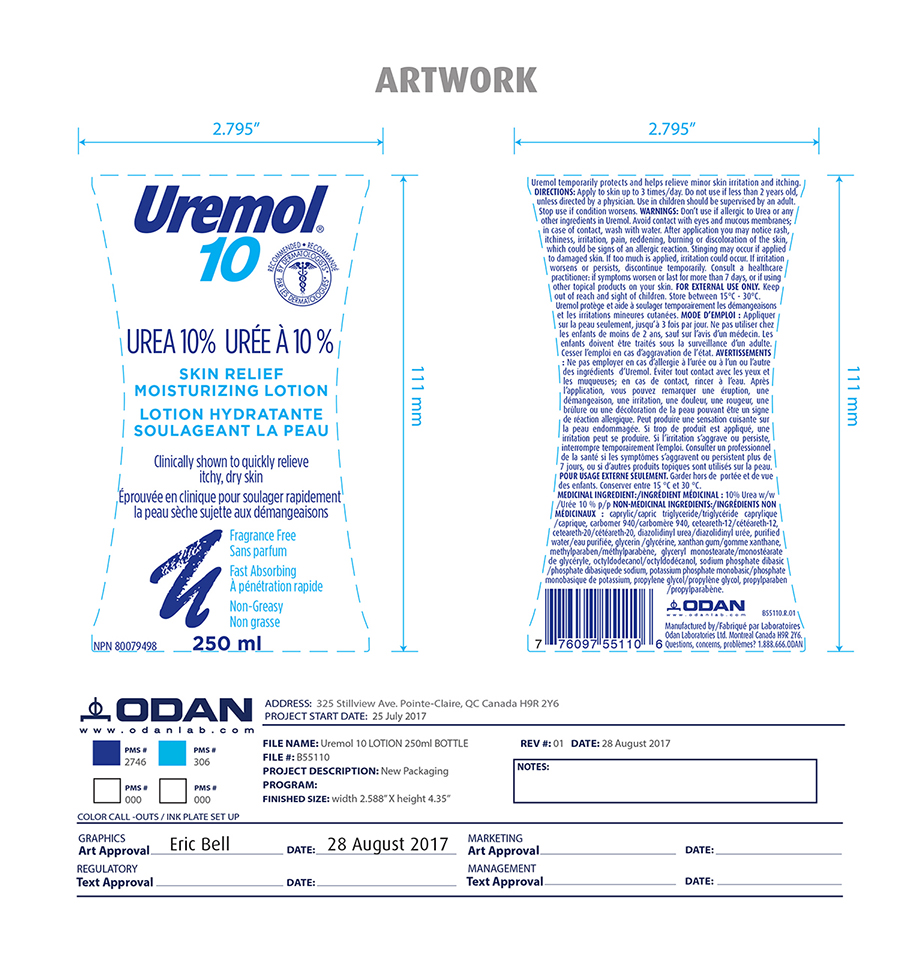
Label: UREMOL- urea lotion
- NDC Code(s): 61344-452-30
- Packager: Odan Laboratories Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Inactive ingredients
- Indication and usage
- PURPOSE
- DOSAGE & ADMINISTRATION
- KEEP OUT OF REACH OF CHILDREN
-
Warning
Don't use if allergic to urea or any other ingredients in Uremol. Avoid contact with eyes and mucous membrane; in case of contact, wash with water. After application you may notice rash, itchiness, irritation, pain, reddening, burning or discoloration of the skin, which could be sign of an allergic reaction. Stinging may occur if applied to damaged skin. If too much is applied, irritation may occur. If irritation worsens or persists, discontinue temporarily. Consult a health care professional if symtoms worsen or last for more than 7 days or if using other topical product on your skin.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UREMOL
urea lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61344-452 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength UREA (UNII: 8W8T17847W) (UREA - UNII:8W8T17847W) UREA 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength POTASSIUM PHOSPHATE, MONOBASIC (UNII: 4J9FJ0HL51) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM PHOSPHATE DIBASIC DIHYDRATE (UNII: 94255I6E2T) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PROPYLPARABEN (UNII: Z8IX2SC1OH) CAPRYLIC/CAPRIC ACID (UNII: DI775RT244) TRICAPRILIN (UNII: 6P92858988) CARBOMER 940 (UNII: 4Q93RCW27E) CETEARETH-12 (UNII: 7V4MR24V5P) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) METHYLPARABEN (UNII: A2I8C7HI9T) OCTYLDODECANOL (UNII: 461N1O614Y) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61344-452-30 250 mL in 1 BOTTLE; Type 6: Drug/Biologic Combination 05/31/2019 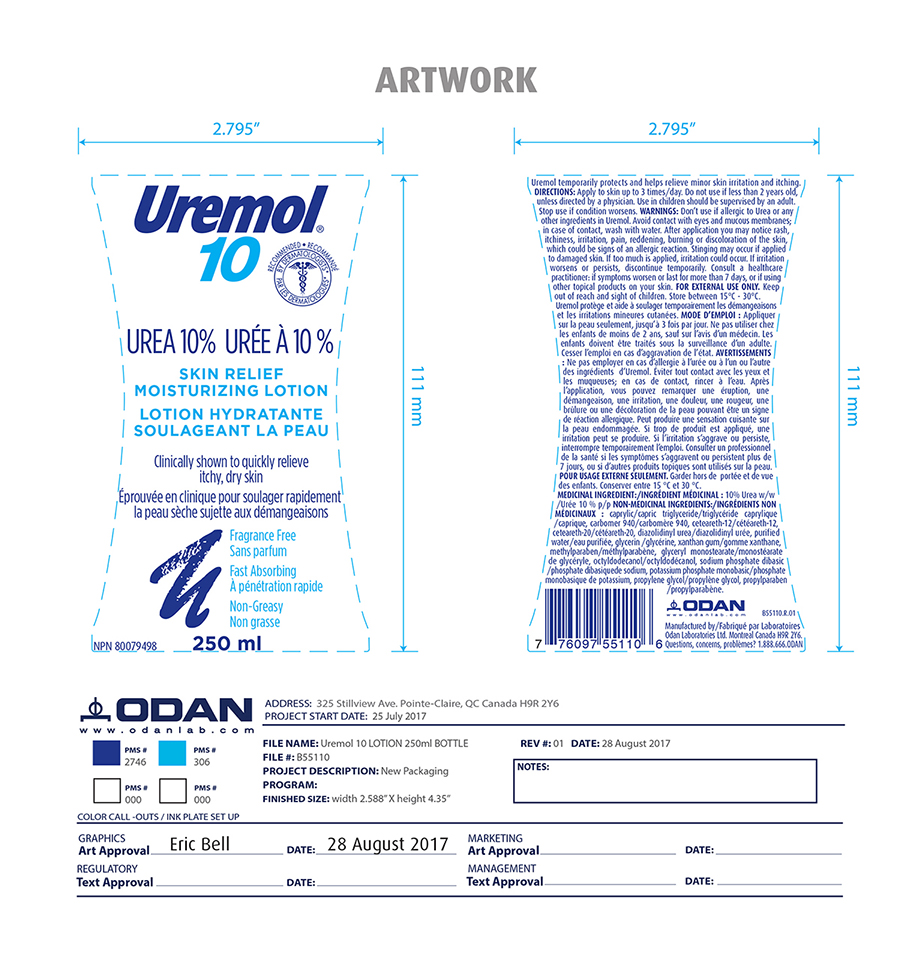
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/31/2019 Labeler - Odan Laboratories Ltd (208585604) Establishment Name Address ID/FEI Business Operations Odan Laboratories LTD 208585604 manufacture(61344-452)