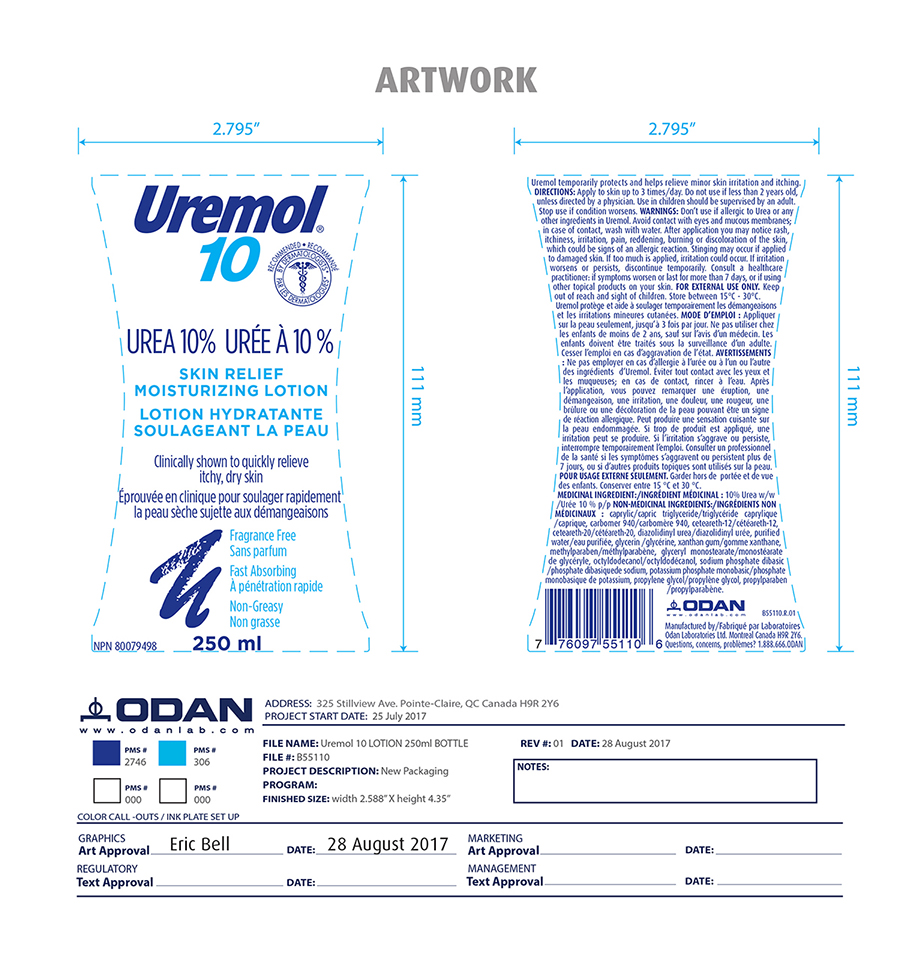
Inactive ingredients
Caprylic, Caprylic triglyceride, carbomer 940, ceteareth-12, ceteareth-20, citrid acid, diazolidinyl urea, purified water, glycerin, xanthan gum, methylparaben, glyceryl monostearate, octyldodecanol, sodium phosphate dibasic, potassium phosphate monobasic, propylene glycol, propylparaben,
Indication and usage
Uremol temporarily protects and helps relieve minor skin irritation and itching. Apply to skin up to 3 times/day. Do not use under 2 years old, unless directed by a physician. Used on children should be supervised by an adult. Stop use if condition worsens.
Uremol 10 % temporarily protects and helps relieve minor skin irritation and itching. Apply to skin up to 3 times/day. Do not use on children under 2 years old.
Warning
Don't use if allergic to urea or any other ingredients in Uremol. Avoid contact with eyes and mucous membrane; in case of contact, wash with water. After application you may notice rash, itchiness, irritation, pain, reddening, burning or discoloration of the skin, which could be sign of an allergic reaction. Stinging may occur if applied to damaged skin. If too much is applied, irritation may occur. If irritation worsens or persists, discontinue temporarily. Consult a health care professional if symtoms worsen or last for more than 7 days or if using other topical product on your skin.