Label: 4314 FIRST AID KIT- 4314 first aid kit
4315 FIRST AID KIT- 4315 first aid kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 0498-0100-01, 0498-0100-02, 0498-0501-00, 0498-0750-35, view more0498-4314-01, 0498-4315-01, 59898-420-36 - Packager: Honeywell Safety Products USA, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 10, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Triple Active ingredient
- Triple Purpose
- Triple Uses
-
Triple
Warnings
For external use only
Allergy alert: do not use if you are allergic to any of the ingredients
Do not use
- in the eyes
- over large areas of the body
- Ask a doctor before use if you have
- a deep or puncture wounds
- animal bites
- serious burns
- Triple Directions
- Triple Other information
- Triple Inactive ingredient
- Triple Questions?
- BZK Wipe Active ingredient
- BZK Wipe Purpose
- BzK Wipe Uses
-
BZK Wipe
Warnings
For external use onlyDo not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
- BZK Wipe Directions
- BZK Wipe Other information
- BZK Wipe Inactive ingredient
- BZK Wipe Questions
- Eyewash Active ingredient
- Eyewassh Purpose
- Eyewash Uses
-
Eyewash
Warnings
For external use only
- Obtain immediate medical treatment for all open wounds in or near eyes.
- To avoid contamination, do not touch tip of container to any surface.
- Do not reuse.
- Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
- Eyeash Directions
- Eyewash Inactive ingredients
- Eyeash Questions
- Hand Sanitizer Active ingredient
- Hand Sanitizer Purpose
- Hand Sanitizer Uses
- Hand Sanitizer Warnings
- Hand Sanitizer Directions
- Hand Sanitizer Other information
- Hand Sanitizer Inactive ingredients
- Hand Sanitizer Questions or Comments?
-
4314
Sf00004528 Kit Contents
1 1X3 PLASTIC 100/BOX
1 TRIPLE ANTIBIOTIC 10 PER
1 EYE DRESS PKT W/4 ADH STRIPS
1 TRIANGULAR BDG, NON-STERILE
2 GAUZE PADS, 3" X 3", 4 PER
1 GAUZE BANDAGE, 2" X 6 YD,2 PER
1 INSTANT COLD PACK 4" X 6"
1 BUFFERED EYE WASH 1 OZ BTL
1 WATER JEL DRESSING 4" X 4"
2 TOPICAL COOLING JEL
1 NITRILE GLOVES 2PR BBP
1 ANTIMCRBL ANTSPTC TWLETTS
1 ADHES TAPE W/P 1"X 2 1/2 YD
1 1FIRST AID GUIDE ASHIEMERGENCY SURVIVAL BLANKET
1 HAND SANITIZER 0.9G WJ 25/BX
2 BLOODSTOPPER
1 CPR FILTERSHIELD 77-100
1 F A KIT EMPTY BLANK 140
1 LBL STOCK 6-3/8"X4"
1 LBL STOCK 4"X2-7/8"
1 SCISSORS ANGLED 1EA IN BAG
-
4315
SF00004529 kit contents
1 1X3 PLASTIC 100/BOX
3 TRIPLE ANTIBIOTIC 10 PER
1 EYE DRESS PKT W/4 ADH STRIPS
1 GAUZE BANDAGE, 4" X 6 YD
1 TOURNIQUET AND FORCEPS 1 EA
2 TRIANGULAR BDG, NON-STERILE
4 GAUZE PADS, 3" X 3", 4 PER
2 GAUZE BANDAGE, 2" X 6 YD,2 PER
2 INSTANT COLD PACK 4" X 6"
2 WATER JEL DRESSING 4" X 4"
5 TOPICAL COOLING JEL
2 NITRILE GLOVES 2PR BBP
5 ANTIMCRBL ANTSPTC TWLETTS
2 ADHES TAPE W/P 1"X 2 1/2 YD
1 FIRST AID GUIDE ASHI
1 EMERGENCY SURVIVAL BLANKET
1 HAND SANITIZER 0.9G WJ 25/BX
4 BLOODSTOPPER
1 CPR FILTERSHIELD 77-100
1 4OZ BFS EYEWASH TRILINGUAL BOTTLE
1 180 EMPTY BLANK NO LOGO
1 SAM SPLINT 4.5"X36" ORNGE/BL
1 LBL STOCK 6-3/8"X4"
1 LBL STOCK 4"X2-7/8"
1 SCISSORS ANGLED 1EA IN BAG
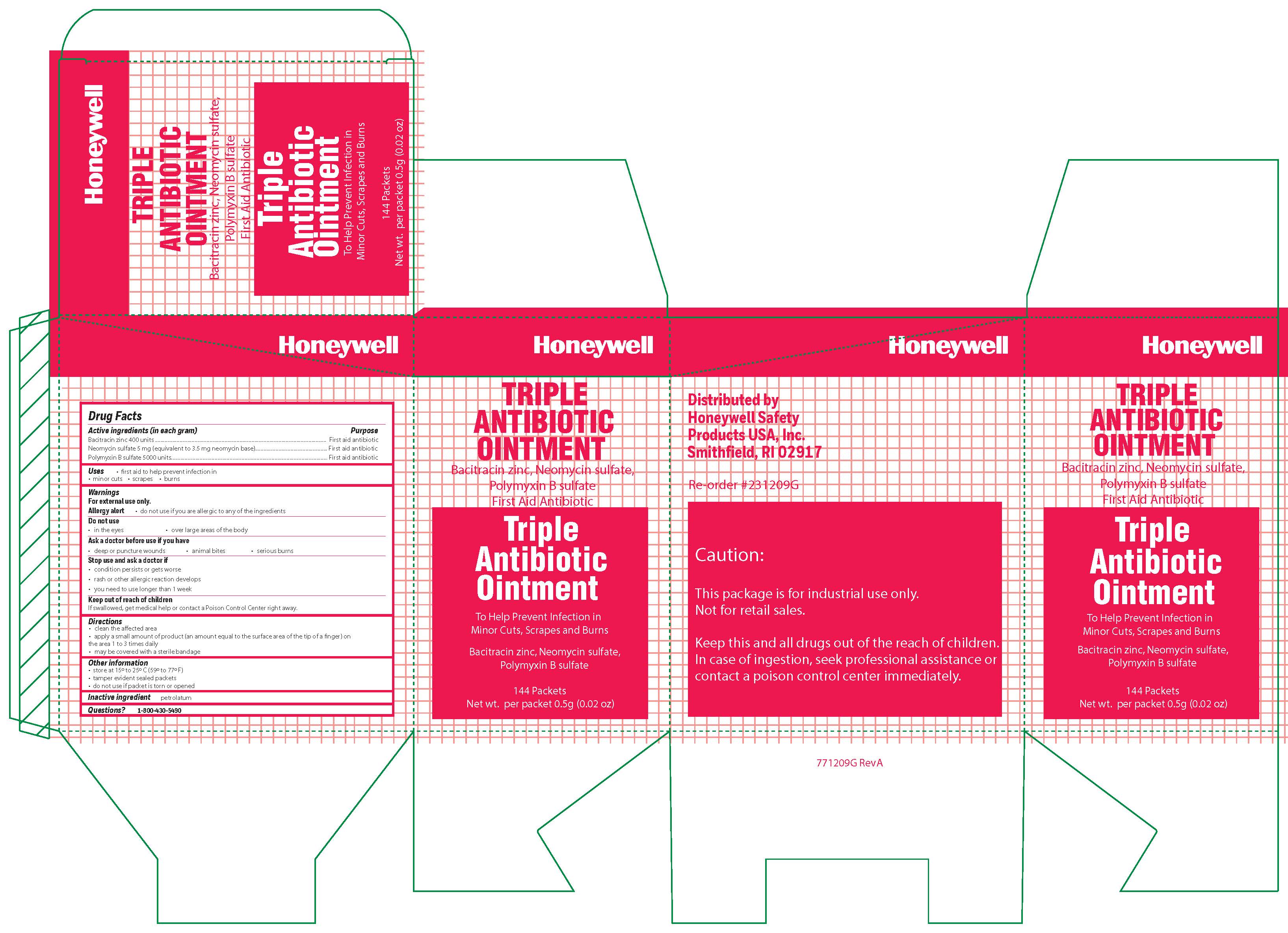
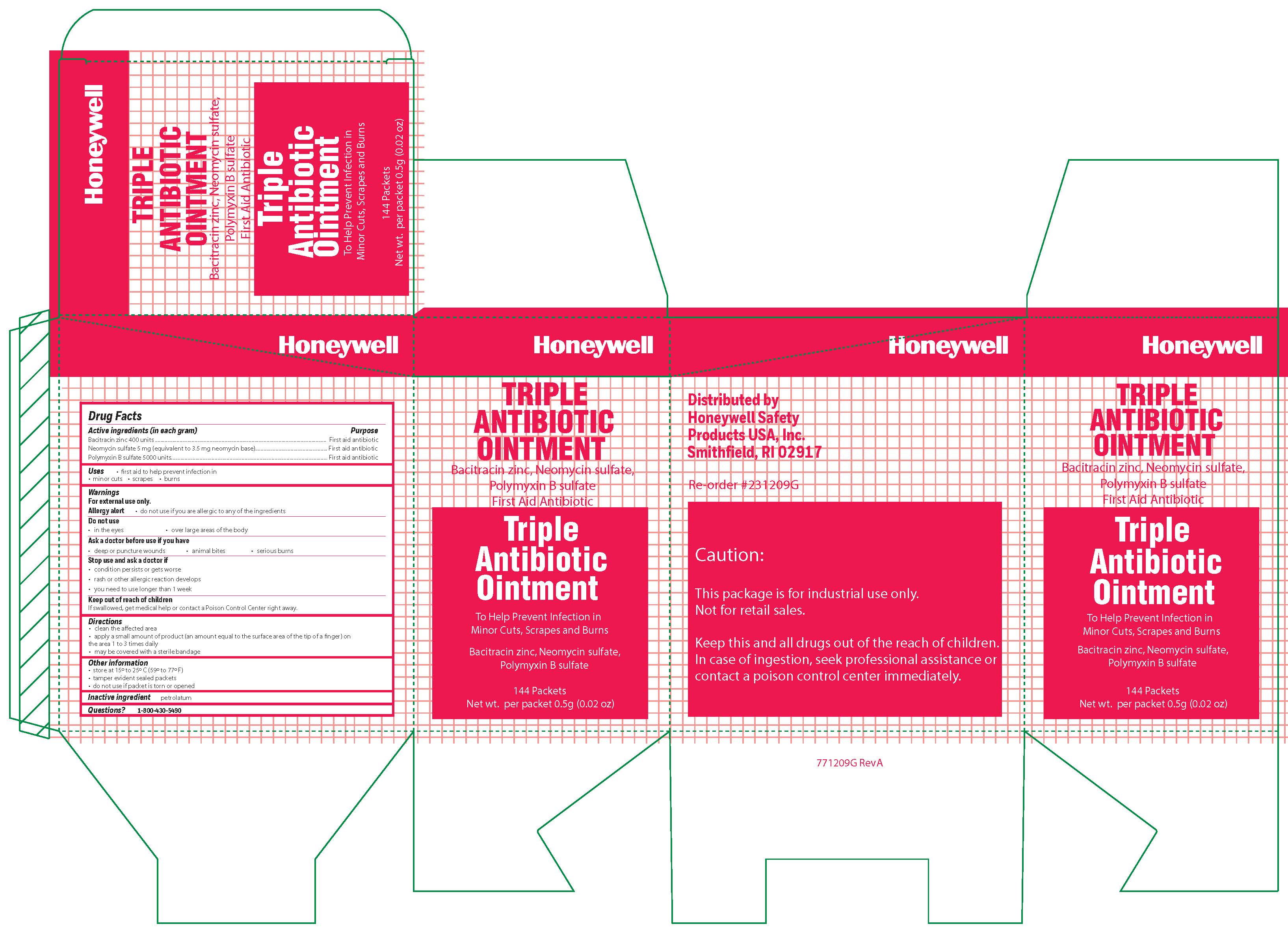
- Triple Principal Display Panel
- BZK Wipe Principal Display Panel
- Eyewash Principal Display Panel
- Hand Sanitizer Principal Display Panel
- 4314 Kit Label SF00004528
- 4315 Kit Label SF00004529
-
INGREDIENTS AND APPEARANCE
4314 FIRST AID KIT
4314 first aid kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0498-4314 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-4314-01 1 in 1 KIT 09/13/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 30 mL Part 2 10 PACKET 9 g Part 3 1 PACKET 1.4 mL Part 4 25 BOTTLE, PLASTIC 22.5 mL Part 1 of 4 EYESALINE EMERGENCY EYEWASH
purified water liquidProduct Information Item Code (Source) NDC:0498-0100 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 98.6 mL in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0100-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 12/18/2018 Part 2 of 4 TRIPLE ANTIBIOTIC
bacitracin zinc, polymyxin b sulfate, neomycin sulfate ointmentProduct Information Item Code (Source) NDC:0498-0750 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 5000 [iU] in 1 g BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 400 [iU] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 3.5 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0750-35 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 09/19/2018 Part 3 of 4 ANTISEPTIC TOWELETTE
benzalkonium chloride liquidProduct Information Item Code (Source) NDC:0498-0501 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0501-00 1.4 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 12/22/2017 Part 4 of 4 INSTANT HAND SANITIZER
alcohol liquidProduct Information Item Code (Source) NDC:59898-420 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 62 mL in 100 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) TRIISOPROPANOLAMINE (UNII: W9EN9DLM98) CARBOMER COPOLYMER TYPE A (UNII: 71DD5V995L) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59898-420-36 0.9 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 04/15/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/13/2018 4315 FIRST AID KIT
4315 first aid kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0498-4315 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-4315-01 1 in 1 KIT 09/13/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 118 mL Part 2 30 PACKET 27 g Part 3 5 PACKET 7 mL Part 4 25 BOTTLE, PLASTIC 22.5 mL Part 1 of 4 EYESALINE EMERGENCY EYEWASH
purified water liquidProduct Information Item Code (Source) NDC:0498-0100 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 98.6 mL in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0100-02 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 12/18/2018 Part 2 of 4 TRIPLE ANTIBIOTIC
bacitracin zinc, polymyxin b sulfate, neomycin sulfate ointmentProduct Information Item Code (Source) NDC:0498-0750 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 5000 [iU] in 1 g BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 400 [iU] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 3.5 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0750-35 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 09/19/2018 Part 3 of 4 ANTISEPTIC TOWELETTE
benzalkonium chloride liquidProduct Information Item Code (Source) NDC:0498-0501 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0501-00 1.4 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 12/22/2017 Part 4 of 4 INSTANT HAND SANITIZER
alcohol liquidProduct Information Item Code (Source) NDC:59898-420 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 62 mL in 100 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) TRIISOPROPANOLAMINE (UNII: W9EN9DLM98) CARBOMER COPOLYMER TYPE A (UNII: 71DD5V995L) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59898-420-36 0.9 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 04/15/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/13/2018 Labeler - Honeywell Safety Products USA, Inc. (079287321) Registrant - Honeywell Safety Products USA, Inc. (079287321) Establishment Name Address ID/FEI Business Operations Honeywell Safety Products USA, Inc 079287321 pack(0498-4314, 0498-4315) Establishment Name Address ID/FEI Business Operations Water-Jel Technologies 155522589 manufacture(0498-0750, 59898-420) Establishment Name Address ID/FEI Business Operations Honeywell Safety Products USA, Inc. 167518617 manufacture(0498-0100) Establishment Name Address ID/FEI Business Operations Changzhou Maokang Medical 421317073 manufacture(0498-0501)