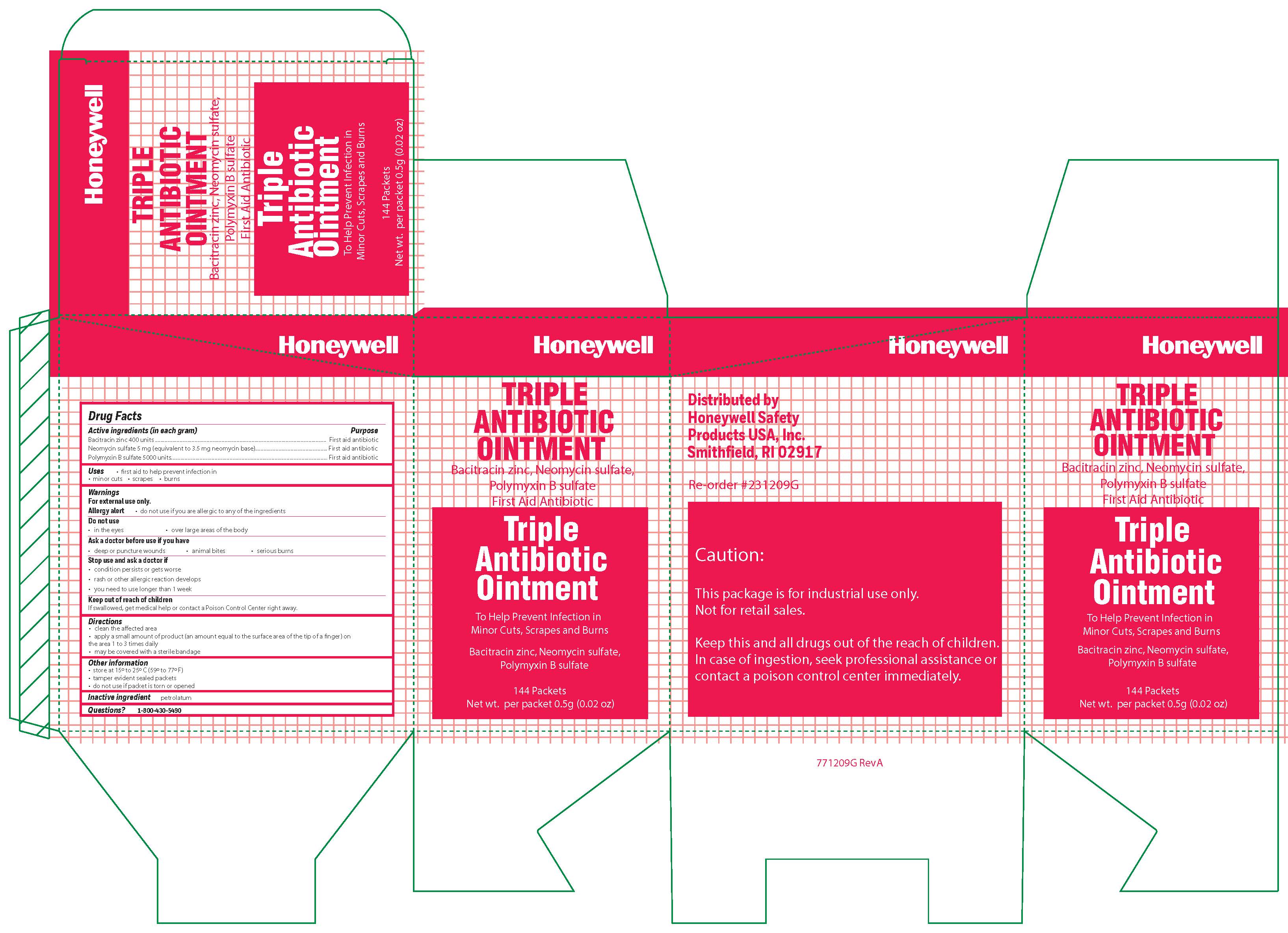
Triple
Active ingredient
Bacitracin zinc 400 units
Neomycin sulfate (5 mg equivalent to 3.5 mg Neomycin base)
Polymyxin B sulfate 5000 units
Triple
Warnings
For external use only
Allergy alert: do not use if you are allergic to any of the ingredients
Do not use
- in the eyes
- over large areas of the body
- Ask a doctor before use if you have
- a deep or puncture wounds
- animal bites
- serious burns
Triple
Directions
- clean the affected area
- apply a small amount of the product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Triple
Other information
- store at 15 0 to 25 0 C (59 0 to 77 0 F)
- tamper evident sealed packets
- do not use if packet is torn or opened
BZK Wipe
Warnings
For external use only
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
BZK Wipe
Other information
- store at room temperature 15 0 to 30 0 C (59 0 - 86 0 F)
- do not reuse towelette
Eyewash
Uses
- for flushing the eye to remove loose foreign material, air pollutants or chlorinated water
Eyewash
Warnings
For external use only
- Obtain immediate medical treatment for all open wounds in or near eyes.
- To avoid contamination, do not touch tip of container to any surface.
- Do not reuse.
- Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
Eyeash
Directions
- remove contacts before using
- twist top to remove
- flush the affected area as needed
- control rate of flow by pressure on the bottle
- if necessary, continue flushing with emergency eyewash or shower
Hand Sanitizer
Warnings
For external use only
Flammable, keep away from fire or flame
Hand Sanitizer
Inactive ingredients
acrylates/C10-30 alkyl acrylate crosspolymer, aloe barbadensis leaf juice, dl-alpha tocopheryl acetate, fragrance, PEG-60 almond glycerides, propylene glycol, purified water, triisopropanolamine
4314
Sf00004528 Kit Contents
1 1X3 PLASTIC 100/BOX
1 TRIPLE ANTIBIOTIC 10 PER
1 EYE DRESS PKT W/4 ADH STRIPS
1 TRIANGULAR BDG, NON-STERILE
2 GAUZE PADS, 3" X 3", 4 PER
1 GAUZE BANDAGE, 2" X 6 YD,2 PER
1 INSTANT COLD PACK 4" X 6"
1 BUFFERED EYE WASH 1 OZ BTL
1 WATER JEL DRESSING 4" X 4"
2 TOPICAL COOLING JEL
1 NITRILE GLOVES 2PR BBP
1 ANTIMCRBL ANTSPTC TWLETTS
1 ADHES TAPE W/P 1"X 2 1/2 YD
1 1FIRST AID GUIDE ASHIEMERGENCY SURVIVAL BLANKET
1 HAND SANITIZER 0.9G WJ 25/BX
2 BLOODSTOPPER
1 CPR FILTERSHIELD 77-100
1 F A KIT EMPTY BLANK 140
1 LBL STOCK 6-3/8"X4"
1 LBL STOCK 4"X2-7/8"
1 SCISSORS ANGLED 1EA IN BAG
4315
SF00004529 kit contents
1 1X3 PLASTIC 100/BOX
3 TRIPLE ANTIBIOTIC 10 PER
1 EYE DRESS PKT W/4 ADH STRIPS
1 GAUZE BANDAGE, 4" X 6 YD
1 TOURNIQUET AND FORCEPS 1 EA
2 TRIANGULAR BDG, NON-STERILE
4 GAUZE PADS, 3" X 3", 4 PER
2 GAUZE BANDAGE, 2" X 6 YD,2 PER
2 INSTANT COLD PACK 4" X 6"
2 WATER JEL DRESSING 4" X 4"
5 TOPICAL COOLING JEL
2 NITRILE GLOVES 2PR BBP
5 ANTIMCRBL ANTSPTC TWLETTS
2 ADHES TAPE W/P 1"X 2 1/2 YD
1 FIRST AID GUIDE ASHI
1 EMERGENCY SURVIVAL BLANKET
1 HAND SANITIZER 0.9G WJ 25/BX
4 BLOODSTOPPER
1 CPR FILTERSHIELD 77-100
1 4OZ BFS EYEWASH TRILINGUAL BOTTLE
1 180 EMPTY BLANK NO LOGO
1 SAM SPLINT 4.5"X36" ORNGE/BL
1 LBL STOCK 6-3/8"X4"
1 LBL STOCK 4"X2-7/8"
1 SCISSORS ANGLED 1EA IN BAG