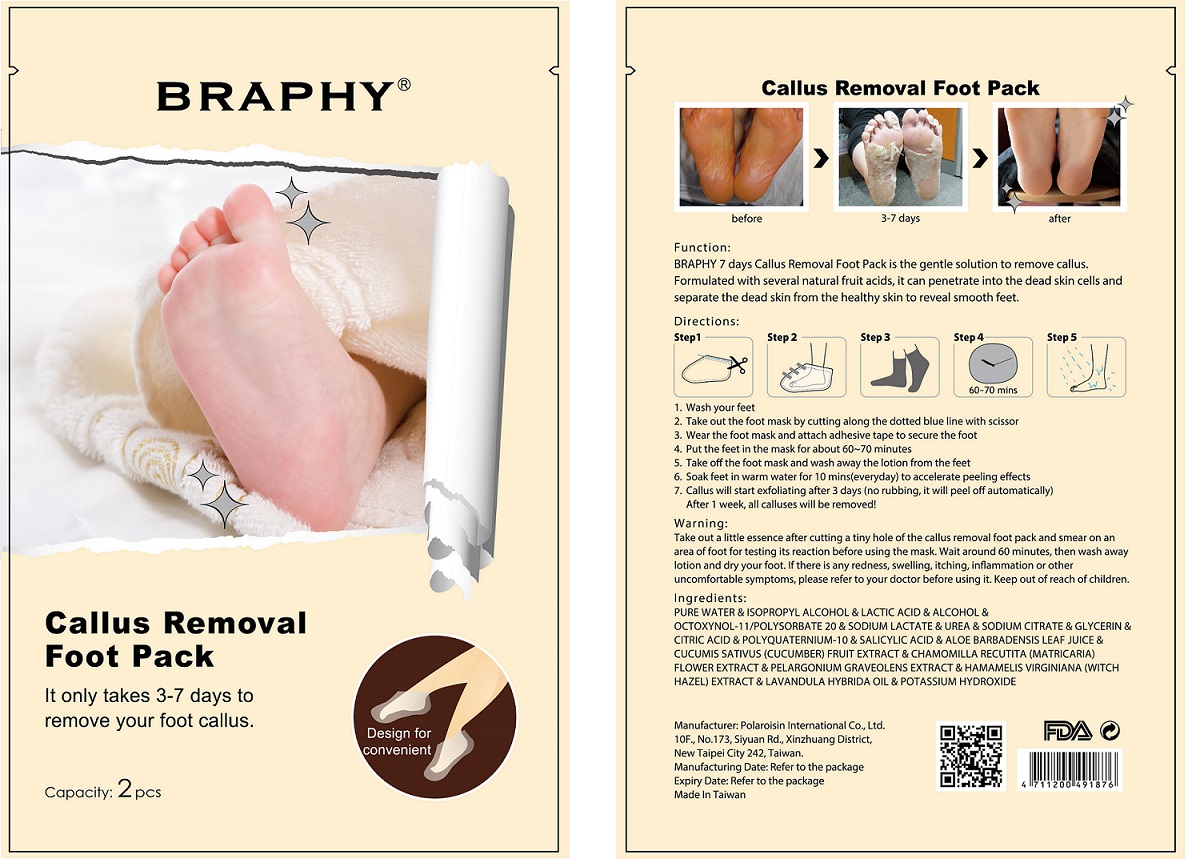
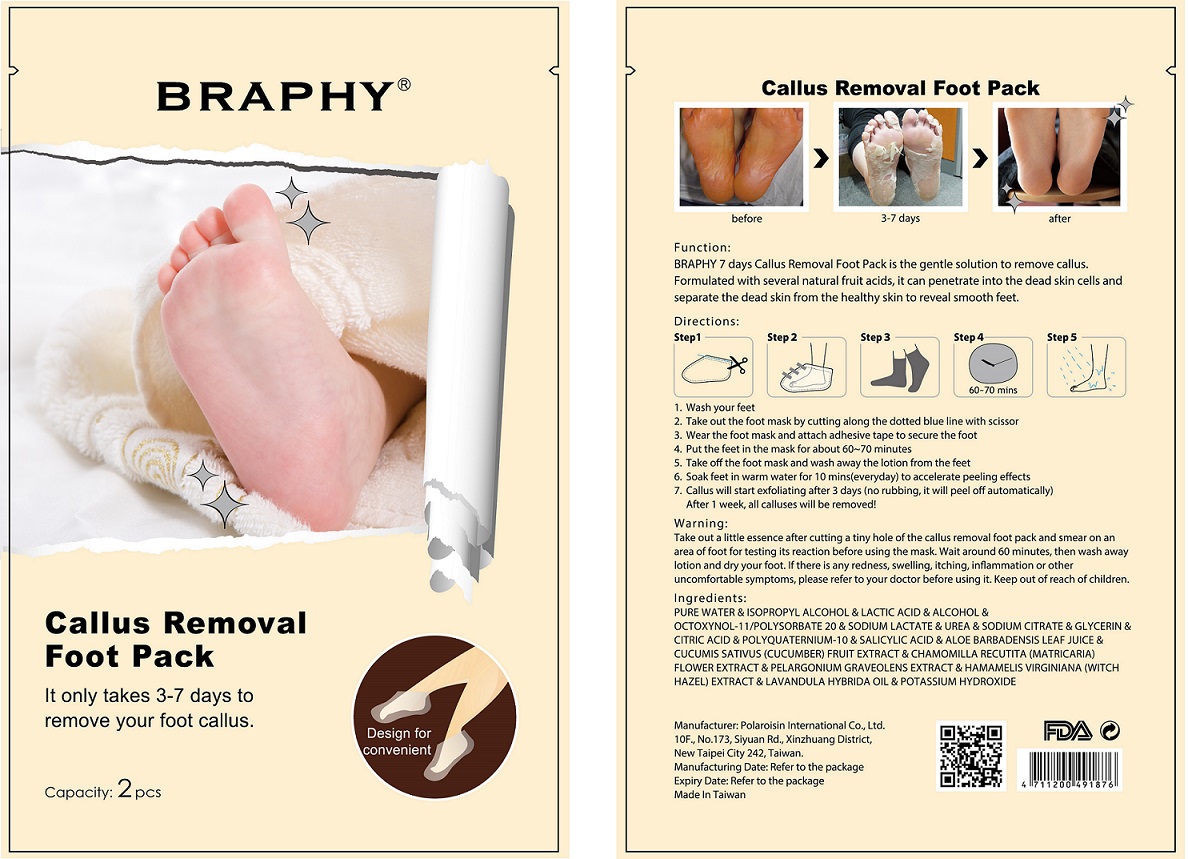
Label: CALLUS REMOVAL FOOT PACK- salicylic acid patch
-
Contains inactivated NDC Code(s)
NDC Code(s): 69264-016-25 - Packager: POLAROISIN INTERNATIONAL CO., LTD.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 17, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- Function:
-
Warning:
Take out a little essence after cutting a tiny hole of the callus removal foot pack and smear on an area of foot for testing its reaction before using the mask. Wait around 60 minutes, then wash away lotion and dry your foot. If there is any redness, swelling, itching, inflammation or other uncomfortable symptoms, please refer to your doctor before using it.
-
Directions:
- Wash your feet
- Take out the foot mask by cutting along the dotted blue line with scissor
- Wear the foot mask and attach adhesive tape to secure the foot
- Put the feet in the mask for about 60-70 minutes
- Take off the foot mask and wash away the lotion from the feet
- Soak feet in warm water for 10 mins(everyday) to accelerate peeling effects
- Callus will start exfoliating after 3 days (no rubbing, it will peel off automatically). After 1 week, all calluses will be removed!
-
Ingredients:
PURE WATER & ISOPROPYL ALCOHOL & LACTIC ACID & ALCOHOL & OCTOXYNOL-11/POLYSORBATE 20 & SODIUM LACTATE & UREA & SODIUM CITRATE & GLYCERIN & CITRIC ACID & POLYQUATERNIUM-10 & SALICYLIC ACID & ALOE BARBADENSIS LEAF JUICE & CUCUMIS SATIVUS (CUCUMBER) FRUIT EXTRACT & CHAMOMILLA RECUTITA (MATRICARIA) FLOWER EXTRACT & PELARGONIUM GRAVEOLENS EXTRACT & HAMAMELIS VIRGINIANA (WITCH HAZEL) EXTRACT & LAVANDULA HYBRIDA OIL & POTASSIUM HYDROXIDE
- SPL UNCLASSIFIED SECTION
- Packaging
-
INGREDIENTS AND APPEARANCE
CALLUS REMOVAL FOOT PACK
salicylic acid patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69264-016 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 11.9 mg in 2 Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISOPROPYL ALCOHOL (UNII: ND2M416302) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALCOHOL (UNII: 3K9958V90M) OCTOXYNOL-11 (UNII: SQL994V0M6) POLYSORBATE 20 (UNII: 7T1F30V5YH) SODIUM LACTATE (UNII: TU7HW0W0QT) UREA (UNII: 8W8T17847W) SODIUM CITRATE (UNII: 1Q73Q2JULR) GLYCERIN (UNII: PDC6A3C0OX) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POLYQUATERNIUM-10 (1000 MPA.S AT 2%) (UNII: GMR4PEN8PK) ALOE VERA LEAF (UNII: ZY81Z83H0X) CUCUMBER (UNII: YY7C30VXJT) CHAMOMILE (UNII: FGL3685T2X) PELARGONIUM GRAVEOLENS FLOWERING TOP (UNII: 1P36QZP48P) HAMAMELIS VIRGINIANA TOP (UNII: UDA30A2JJY) LAVANDIN OIL (UNII: 9RES347CKG) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69264-016-25 2 in 1 BAG; Type 0: Not a Combination Product 12/17/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358F 12/17/2015 Labeler - POLAROISIN INTERNATIONAL CO., LTD. (658727511) Establishment Name Address ID/FEI Business Operations POLAROISIN INTERNATIONAL CO., LTD. 658727511 manufacture(69264-016)