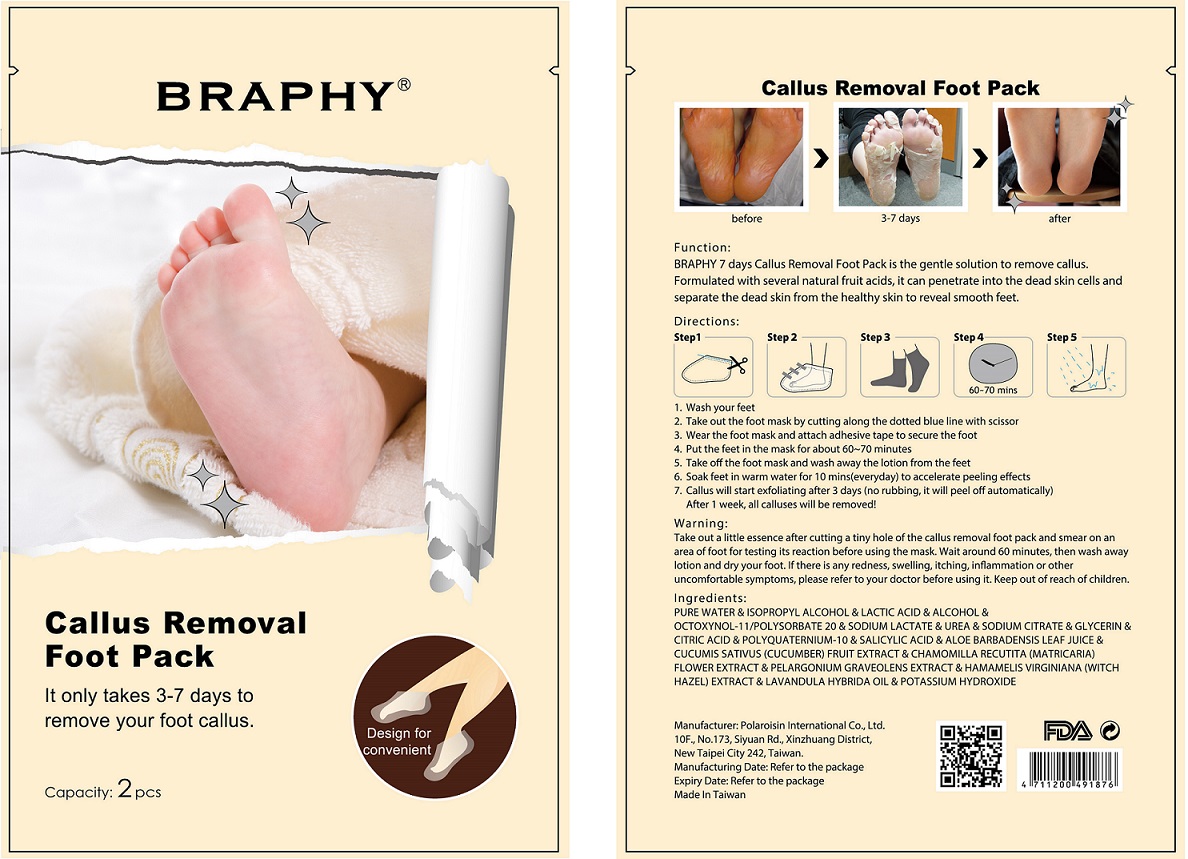
Function:
BRAPHY 7 days Callus Removal Foot Pack is the gentle solution to remove callus. Formulated with several natural fruit acids, it can penetrate into the dead skin cells and separate the dead skin from the healthy skin to reveal smooth feet
Warning:
Take out a little essence after cutting a tiny hole of the callus removal foot pack and smear on an area of foot for testing its reaction before using the mask. Wait around 60 minutes, then wash away lotion and dry your foot. If there is any redness, swelling, itching, inflammation or other uncomfortable symptoms, please refer to your doctor before using it.
Directions:
- Wash your feet
- Take out the foot mask by cutting along the dotted blue line with scissor
- Wear the foot mask and attach adhesive tape to secure the foot
- Put the feet in the mask for about 60-70 minutes
- Take off the foot mask and wash away the lotion from the feet
- Soak feet in warm water for 10 mins(everyday) to accelerate peeling effects
- Callus will start exfoliating after 3 days (no rubbing, it will peel off automatically). After 1 week, all calluses will be removed!
Ingredients:
PURE WATER & ISOPROPYL ALCOHOL & LACTIC ACID & ALCOHOL & OCTOXYNOL-11/POLYSORBATE 20 & SODIUM LACTATE & UREA & SODIUM CITRATE & GLYCERIN & CITRIC ACID & POLYQUATERNIUM-10 & SALICYLIC ACID & ALOE BARBADENSIS LEAF JUICE & CUCUMIS SATIVUS (CUCUMBER) FRUIT EXTRACT & CHAMOMILLA RECUTITA (MATRICARIA) FLOWER EXTRACT & PELARGONIUM GRAVEOLENS EXTRACT & HAMAMELIS VIRGINIANA (WITCH HAZEL) EXTRACT & LAVANDULA HYBRIDA OIL & POTASSIUM HYDROXIDE