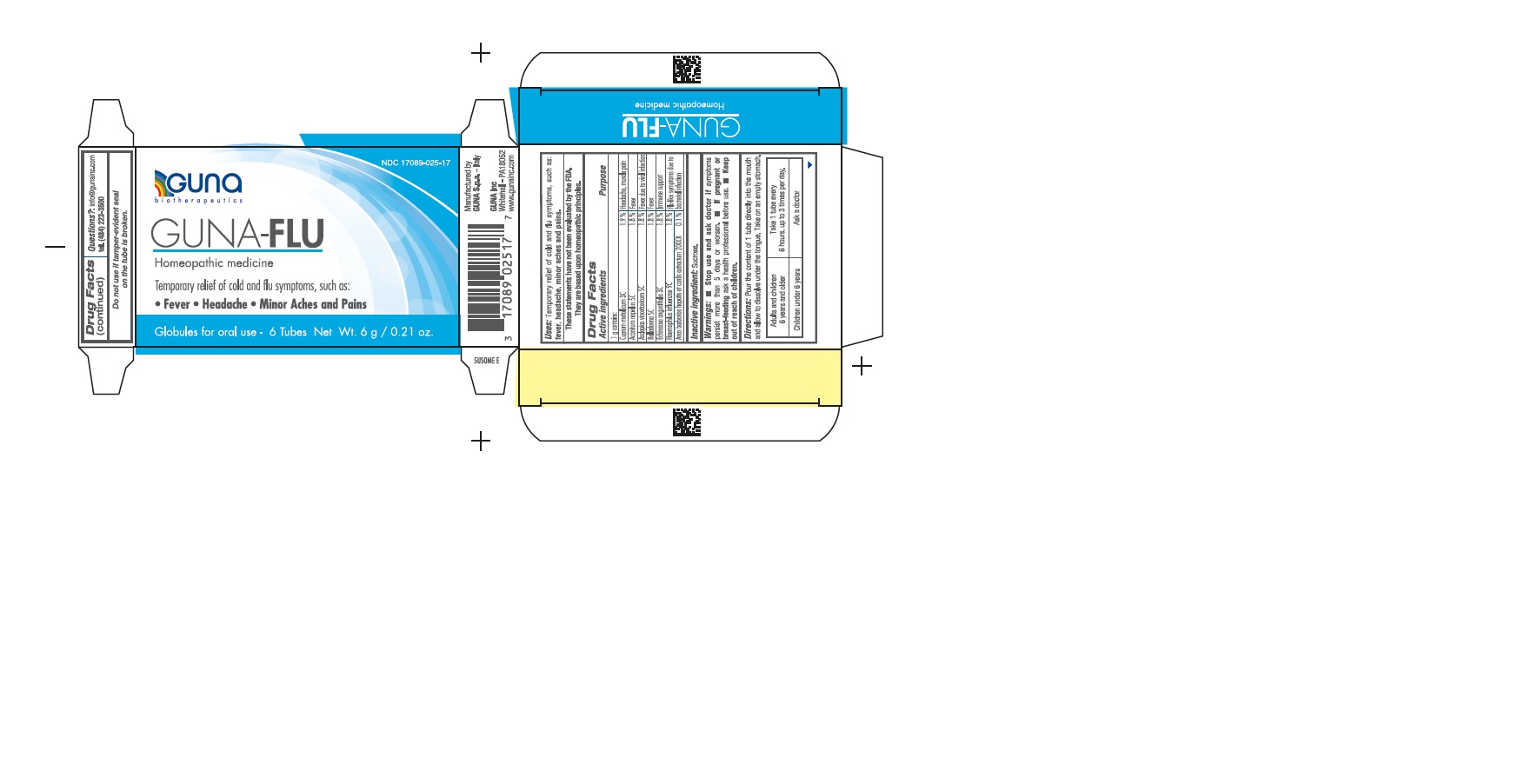
Label: GUNA-FLU- aconitum napellus - asclepias curassavica - atropa belladonna - cairina moschata heart/liver autolysate - copper - echinacea angustifolia - haemophilus influenzae type b - pellet
- NDC Code(s): 17089-025-17
- Packager: Guna spa
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated November 14, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTS/PURPOSE
ACONITUM NAPELLUS 5C FEVER
ANAS BARBARIAE 200CK FLU-LIKE SYMPTOMS DUE TO BACTERIAL INFECTIONS
ASCLEPIAS VINCETOXICUM 5C FEVER DUE TO VIRAL INFECTIONS
BELLADONNA 5C FEVER
CUPRUM METALLICUM 3C HEADACHE, MUSCLE PAIN
ECHINACEA ANGUSTIFOLIA 3C IMMUNE SUPPORT
HAEMOPHILUS INFLUENZAE 9C FLU-LIKE SYMPTOMS DUE TO BACTERIAL INFECTIONS - USES
- WARNINGS
- PREGNANCY
- WARNINGS
-
DIRECTIONS
Take on an empty stomach.
Adults and children 12 years and older Take 1 tube contents to be dissolved in the mouth, every 6 hours, up to 3 times per day.
Children between 12 years and 6 years of age. Take 1 tube contents to be dissolved into a little water, every 6 hours, up to 3 times per day.
Children under 6 years Consult a physician.
- QUESTIONS
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- KEEP OUT OF REACH OF CHILDREN
- WARNINGS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-FLU
aconitum napellus - asclepias curassavica - atropa belladonna - cairina moschata heart/liver autolysate - copper - echinacea angustifolia - haemophilus influenzae type b - pelletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-025 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACONITUM NAPELLUS (UNII: U0NQ8555JD) (ACONITUM NAPELLUS - UNII:U0NQ8555JD) ACONITUM NAPELLUS 5 [hp_C] in 1 g CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE (UNII: RN2HC612GY) (CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE - UNII:RN2HC612GY) CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE 7 [hp_C] in 1 g ASCLEPIAS CURASSAVICA (UNII: JSZ93E47EP) (ASCLEPIAS CURASSAVICA - UNII:JSZ93E47EP) ASCLEPIAS CURASSAVICA 5 [hp_C] in 1 g ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 5 [hp_C] in 1 g COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 3 [hp_C] in 1 g ECHINACEA ANGUSTIFOLIA (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA 3 [hp_C] in 1 g HAEMOPHILUS INFLUENZAE TYPE B (UNII: F2TW0N64FI) (HAEMOPHILUS INFLUENZAE TYPE B - UNII:F2TW0N64FI) HAEMOPHILUS INFLUENZAE TYPE B 9 [hp_C] in 1 g Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) 0.8 g in 1 g Product Characteristics Color white (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-025-17 6 in 1 BOX 05/23/2006 1 1 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/23/2006 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-025)