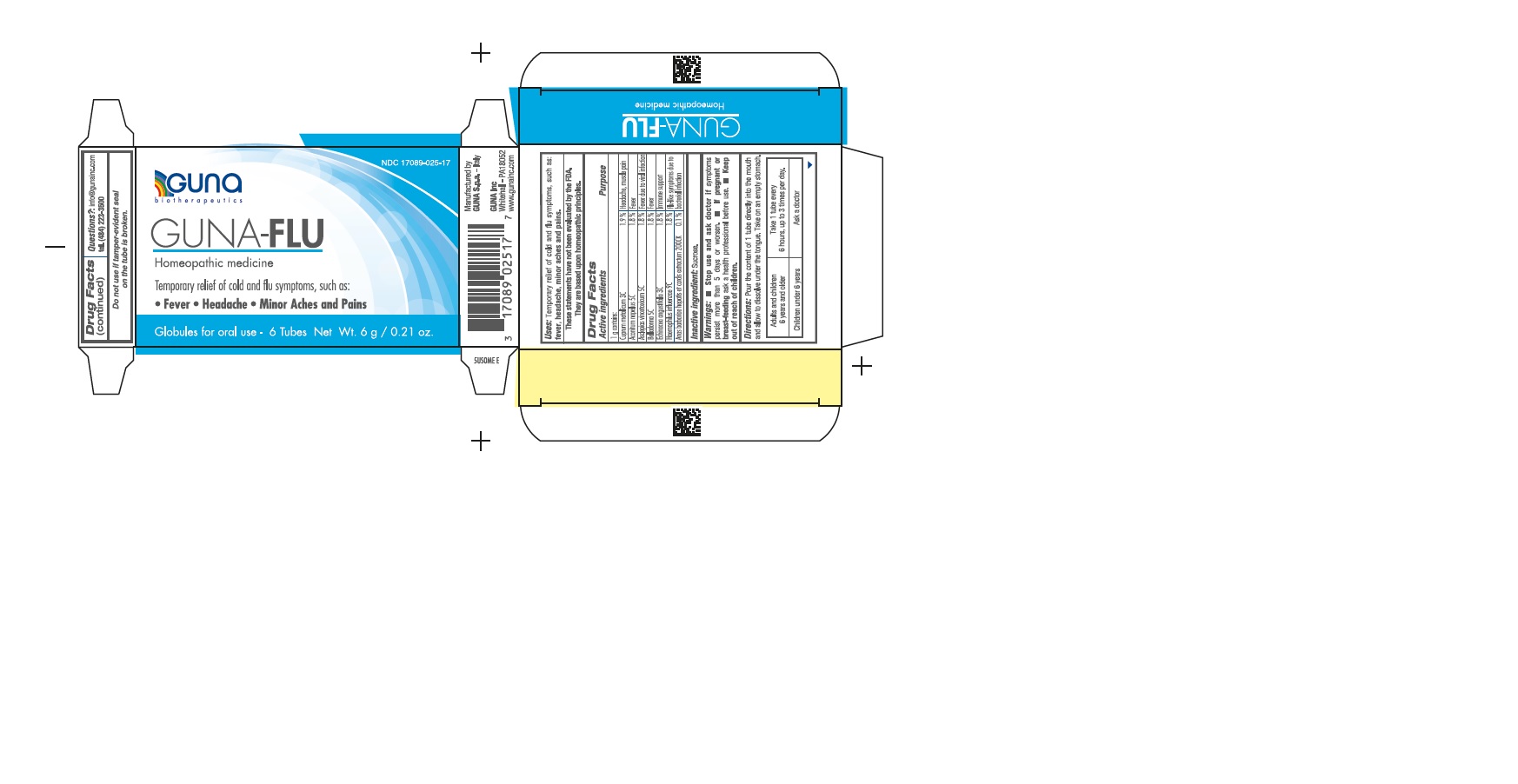
ACTIVE INGREDIENTS/PURPOSE
ACONITUM NAPELLUS 5C FEVER
ANAS BARBARIAE 200CK FLU-LIKE SYMPTOMS DUE TO BACTERIAL INFECTIONS
ASCLEPIAS VINCETOXICUM 5C FEVER DUE TO VIRAL INFECTIONS
BELLADONNA 5C FEVER
CUPRUM METALLICUM 3C HEADACHE, MUSCLE PAIN
ECHINACEA ANGUSTIFOLIA 3C IMMUNE SUPPORT
HAEMOPHILUS INFLUENZAE 9C FLU-LIKE SYMPTOMS DUE TO BACTERIAL INFECTIONS
USES
- Uses: Temporary relief of cold and flu symptoms, such as: fever, headache, minor aches and pains.
WARNINGS
- Stop use and ask doctor if symptoms persist more than 5 days or worsen.
- If pregnant or breast-feeding ask a health professional before use.
- Keep out of reach of children.
DIRECTIONS
Take on an empty stomach.
Adults and children 12 years and older Take 1 tube contents to be dissolved in the mouth, every 6 hours, up to 3 times per day.
Children between 12 years and 6 years of age. Take 1 tube contents to be dissolved into a little water, every 6 hours, up to 3 times per day.
Children under 6 years Consult a physician.
- Adults and children 6 years and older: take 1 tube every 6 hours, up to 3 times per day.
- Children under 6 years: Ask a doctor.
- Directions: pour the content of 1 tube directly into the mouth and allow to dissolve under the tongue. Take on an empty stomach.