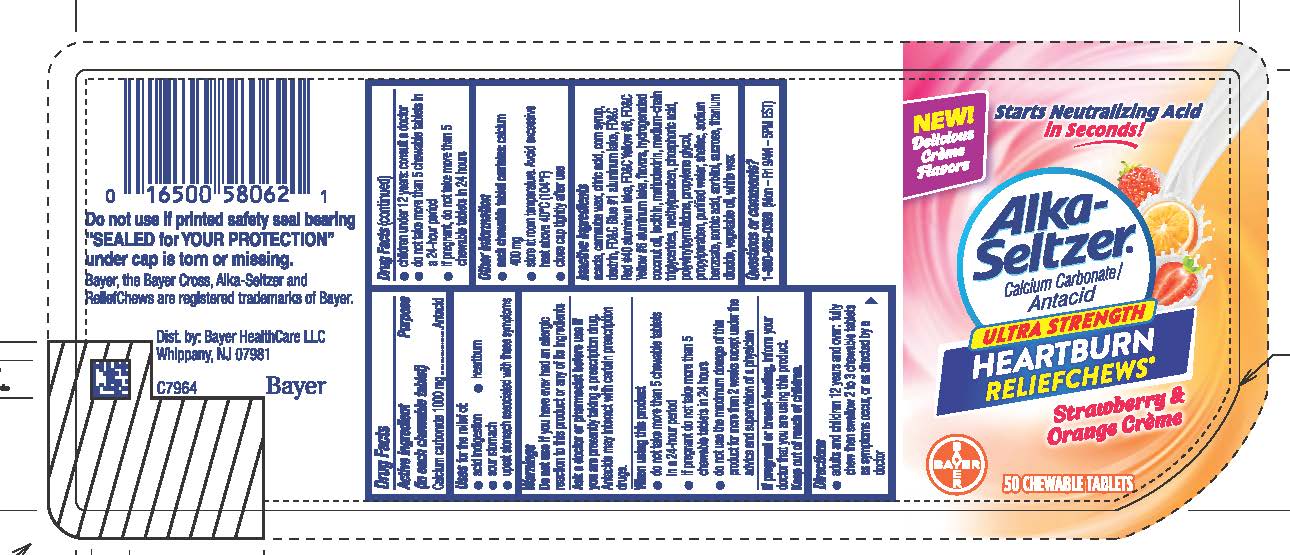
Label: ALKA-SELTZER ULTRA STRENGTH HEARTBURN RELIEFCHEWS STRAWBERRY AND ORANGE- calcium carbonate tablet, chewable
- NDC Code(s): 0280-0059-50
- Packager: Bayer HealthCare LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- Uses
-
WARNINGS
Warnings
Do not use if you have ever had an allergic reaction to this product or any of its ingredientsAsk a doctor or pharmacist before use if you are presently taking a prescription drug. Antacids may interact with certain prescription drugs.
-
Directions
Directions
● adults and children 12 years and over: fully chew then swallow 2 to 3 chewable tablets as symptoms occur, or as directed by a doctor
● children under 12 years: consult a doctor
● do not take more than 5 chewable tablets in a 24-hour period
● if pregnant, do not take more than 5 chewable tablets in 24
hours
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Inactive ingredients acacia, carnauba wax, citric acid, corn syrup, dextrin, FD&C Blue #1 aluminum lake, FD&C Red #40 aluminum lake, FD&C Yellow #6, FD&C Yellow #6 aluminum lake, flavors, hydrogenated coconut oil, lecithin, maltodextrin, medium-chain triglycerides, methylparaben, phosphoric acid, polyvinylpyrrolidone, propylene glycol, propylparaben, purified water, shellac, sodium benzoate, sorbic acid, sorbitol, sucrose, titanium dioxide, vegetable oil, white wax
- Questions or comments
- 50 Tablet bottle label
-
INGREDIENTS AND APPEARANCE
ALKA-SELTZER ULTRA STRENGTH HEARTBURN RELIEFCHEWS STRAWBERRY AND ORANGE
calcium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0280-0059 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CARBONATE 1000 mg Inactive Ingredients Ingredient Name Strength ACACIA (UNII: 5C5403N26O) CORN SYRUP (UNII: 9G5L16BK6N) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) MALTODEXTRIN (UNII: 7CVR7L4A2D) METHYLPARABEN (UNII: A2I8C7HI9T) SHELLAC (UNII: 46N107B71O) SORBIC ACID (UNII: X045WJ989B) WHITE WAX (UNII: 7G1J5DA97F) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) HYDROGENATED COCONUT OIL (UNII: JY81OXM1OM) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SUCROSE (UNII: C151H8M554) CARNAUBA WAX (UNII: R12CBM0EIZ) FD&C BLUE NO. 1--ALUMINUM LAKE (UNII: J9EQA3S2JM) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PROPYLPARABEN (UNII: Z8IX2SC1OH) PHOSPHORIC ACID (UNII: E4GA8884NN) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SORBITOL (UNII: 506T60A25R) Product Characteristics Color red, orange Score no score Shape ROUND Size 19mm Flavor ORANGE, STRAWBERRY Imprint Code U2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0280-0059-50 50 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/03/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 03/03/2019 Labeler - Bayer HealthCare LLC. (112117283)