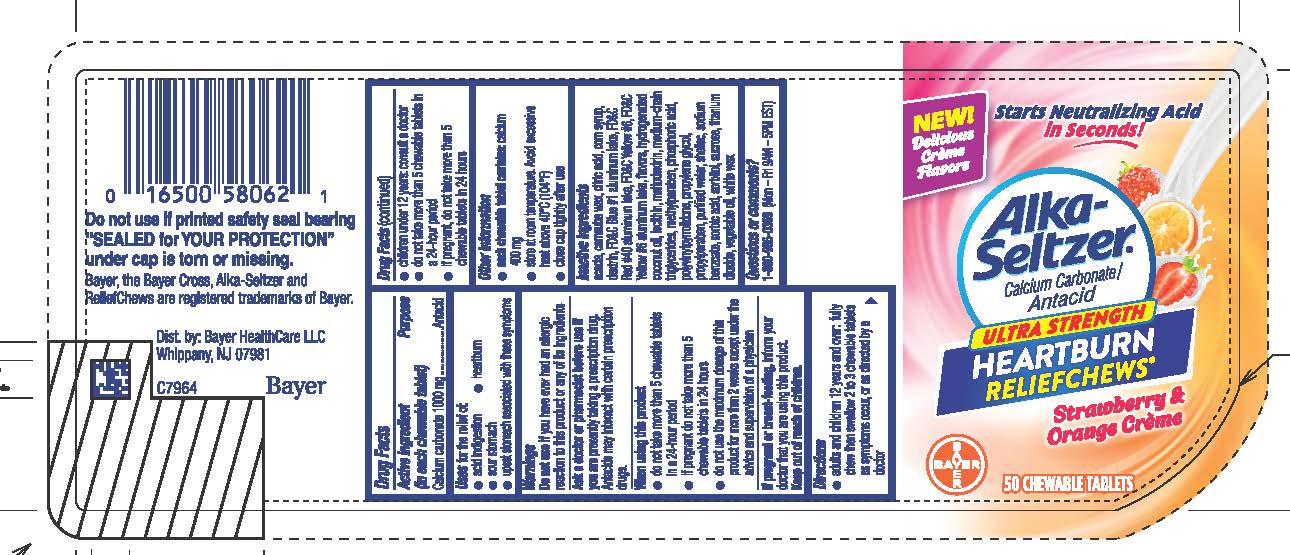
Uses
Uses for the relief of:
● acid indigestion ● heartburn ● sour stomach
● upset stomach associated with these symptoms
Warnings
Do not use if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor or pharmacist before use if you are presently taking a prescription drug. Antacids may interact with certain prescription drugs.
Directions
Directions
● adults and children 12 years and over: fully chew then swallow 2 to 3 chewable tablets as symptoms occur, or as directed by a doctor
● children under 12 years: consult a doctor
● do not take more than 5 chewable tablets in a 24-hour period
● if pregnant, do not take more than 5 chewable tablets in 24
hours
Other information
● each chewable tablet contains: calcium 400 mg
● store at room temperature. Avoid excessive heat above 40°C
(104°F).
● close cap tightly after use
Inactive ingredients acacia, carnauba wax, citric acid, corn syrup, dextrin, FD&C Blue #1 aluminum lake, FD&C Red #40 aluminum lake, FD&C Yellow #6, FD&C Yellow #6 aluminum lake, flavors, hydrogenated coconut oil, lecithin, maltodextrin, medium-chain triglycerides, methylparaben, phosphoric acid, polyvinylpyrrolidone, propylene glycol, propylparaben, purified water, shellac, sodium benzoate, sorbic acid, sorbitol, sucrose, titanium dioxide, vegetable oil, white wax